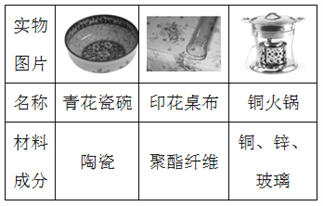
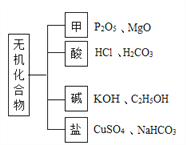
��Ŀ����
����Ŀ���ŷŵķ�ˮ�к������������ͭ������Ⱦ�Ϊ�ⶨ�÷�ˮ�и���Ⱦ��ĺ�������ұͭ���ṩ������ˮ�IJο�������С���ͬѧ����������ʵ�顣ȡ��ˮ500g�������м���������������Ϊ20%������������Һ����ó��������������������������Һ��������ϵ��ͼ������ͼ���������ݼ��㣺
(1)��Ӧ���ɵ�Cu(OH)2������Ϊ________g��
(2)�÷�ˮ���������������������___________��(��д���������)
(3)��ˮ���������������Ϊ________g��������120g����������Һǡ����ȫ��Ӧ��������Һ�����ʵ�����������________��(�����ȷ��0.1%)
���𰸡� 19.6 �⣺��NaOH��Һ������Ϊx�����������Ƶ�����Ϊy��
CuSO4��2NaOH===Cu(OH)2����Na2SO4
����������80��������98����������142
��������x��20%����19.6g��������y
![]() ��
��![]() ��
��![]() ��x��80g��y��28.4g
��x��80g��y��28.4g
�����ᷴӦ��NaOH������Ϊ(120g��80g)��20%��8g��
��500g�÷�ˮ��H2SO4������Ϊz�����������Ƶ�����Ϊm��
H2SO4��2NaOH===Na2SO4��2H2O
��98������80��������142
��z���� ��8g��������m
![]() ��
��![]() ��
��![]() ��z��9.8g��m��14.2g
��z��9.8g��m��14.2g
�÷�ˮ���������������������![]() ��100%��1.96%��
��100%��1.96%��
�𣺸÷�ˮ�������������������Ϊ1.96%�� 9.8�� 7.1%
��������������ѧ֪ʶ��������Ϣ֪��(1)��Ӧ���ɵ�Cu(OH)2������Ϊ19.6g��
(2) �⣺��NaOH��Һ������Ϊx�����������Ƶ�����Ϊy��
CuSO4��2NaOH===Cu(OH)2����Na2SO4
80 98 142
x��20% 19.6g y
![]() ��
��![]() ��
��![]() ��x��80g��y��28.4g
��x��80g��y��28.4g
�����ᷴӦ��NaOH������Ϊ(120g��80g)��20%��8g��
��500g�÷�ˮ��H2SO4������Ϊz�����������Ƶ�����Ϊm��
H2SO4��2NaOH==Na2SO4��2H2O
98 80 142
Z 8g m
![]() ��
��![]() ��
��![]() ��z��9.8g��m��14.2g
��z��9.8g��m��14.2g
�÷�ˮ���������������������![]() ��100%��1.96%��
��100%��1.96%��
�ǵ�����120g����������Һǡ����ȫ��Ӧ��������Һ�����ʵ����������ǡ�
![]() ��100����7.1%
��100����7.1%
���(1)��Ӧ���ɵ�Cu(OH)2������Ϊ19.6g��
(2)�÷�ˮ���������������������1.96%��
(3)��ˮ���������������Ϊ9.8g,��������120g����������Һǡ����ȫ��Ӧ��������Һ�����ʵ�����������7.1%��
�㾦������һ���ۺϼ����⣬���Ӧ�������غ㶨�ɲ����ݻ�ѧ����ʽ���м��㣬�Ƕ�ѧ���Ļ���Ҫ�����ռ��㷽���ͼ��㼼�ɴӶ���߽���Ч�ʡ�

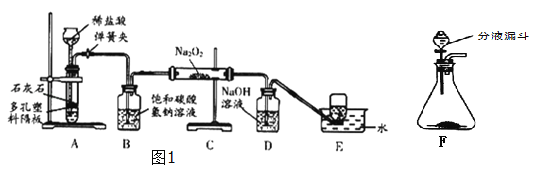
����Ŀ��̽��ѧϰС����ʵ��������ͼ1��ʾװ����ȡCO2��̽��CO2���������(Na2O2)�ķ�Ӧ��
�������ϵ�֪����������(Na2O2)��һ�ֵ���ɫ���壬����CO2��H2O������Ӧ��Na2O2+2CO2�T2Na2CO3+O2��2Na2O2+H2O�T4NaOH+O2��
��ش��������⣺
(1)װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ________________________������װ��A��ȡCO2���ŵ���___________________________________��
(2)װ��B������������CO2�л��е�HCl��������Ӧ��ѧ����ʽΪ____________________________
(3)װ��D��������_______________________��
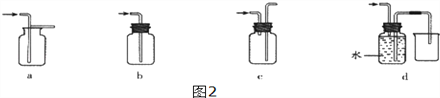
(4)װ��E���ռ�����������____(�ѧʽ)�������廹����������ͼ2װ���е�______�����ռ�(��д��ĸ���)��
(5)Ϊ̽����Ӧ��װ��CӲ�ʲ������й���ijɷ֣���С��ͬѧ��������ͼ3ʵ�飺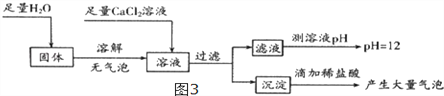
���ݹ����ˮ�ܽ������ݣ��ó�__________________________���ۣ����������Ȼ���Ŀ��Ϊ
_______________________________________�������жϣ���Ӧ��װ��CӲ�ʲ������й���Ϊ
______________________��
(6)����ʵ������ȡ������̼���о��У�̽��ѧϰС���ֽ���������ʵ�飺
ҩƷ��� | �� | �� | �� | �� |
����ʯ | m g����״ | m g����״ | m g����ĩ״ | m g����ĩ״ |
����(����) | w g��ϡ���� | w g��Ũ���� | w g��ϡ���� | w g��Ũ���� |
I.��Ҫ�о�����Ũ�ȴ�С�Է�Ӧ��Ӱ�죬��ѡ��ʵ�����______����(ѡ��ʵ����)��
II..�������Ũ���⣬����ʵ���о�����һ��Ӱ�췴Ӧ��������_____________________��
III.�о��������Ũ��Խ����������ٶ�Խ�죬��ױȽϣ��Զ�������ȷ����______(ѡ����)
A����Ӧ��Ϊ���� B������ʣ����Һ��������С
C�������Ķ�����̼���������� D����ĩ״����ʯ�����ʸ���
���±��е�����ʵ�飬������ԭ��״̬������װ�õȷ�����ڲ��죬ȴ���ܿ��������ƽ�ȵز��������ʵ��Ŀ�ġ�ԭ����ԭ�ϡ�װ�á������ȷ���˼���������ÿ��ʵ���������ƽ�Ȳ���������Ҫ��һ��ԭ��
Ŀ�� | ԭ�� | ����װ�� | �����ƽ�Ȳ����� ����Ҫ��һ��ԭ�� |
��ȡ������̼ | ��״����ʯϡ���� | A | ______________________ |
��ȡ���� | ��ĩ״������̼3%�Ĺ���������Һ | F | ________________________ |