��Ŀ����
����Ŀ��2016����Լ���˻���������������֬����������ɣ���������������������ˡ��˶���������������
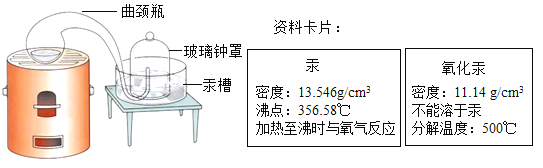
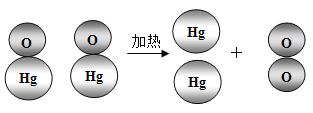
��1����ҵ�ϳ���ͨ��ֽ���������Al2O3���Ʊ���������ͬʱ��������������÷�Ӧ�Ļ�ѧ����ʽΪ________���÷�Ӧ�Ļ�������Ϊ______��
��2��������������Ͻ����������ڻ�����ղػ�档��ԭ�������ڿ��������������γ�һ��___________����������Ʒ����������ʴ�����㻹˵���������Ͻ���ϵ������ŵ㣨����һ�㣩___________________��
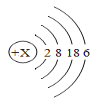
��3���ɼ�����ƥ��ʥ���ڰ���ƥ�˵ĺ�������ǰ���ð��澵������������ȼ���֡�����Ϊ���ֵ�ȼ���ṩ��_________�����ȼ��ʱ��_______��ת��Ϊ���ܺ��ܡ�
��4�����ȼ��ϵͳ��װ������ȼ�ϱ��飨C3H8 ������������_____��ѡ���������л����������������ȼ����������Ӧ�Ļ�ѧ����ʽΪ__________������ȼ�Ϸ��ϡ���ɫ���ˡ���Ҫ��ԭ����____________��
���𰸡�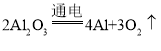 �ֽⷴӦ ���ܵ���������Ĥ �ܶ�С��㡢Ӳ�Ƚϴ�� �㹻������ ��ѧ �л�
�ֽⷴӦ ���ܵ���������Ĥ �ܶ�С��㡢Ӳ�Ƚϴ�� �㹻������ ��ѧ �л� 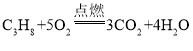 ����ˮ�Ͷ�����̼����Ⱦ����
����ˮ�Ͷ�����̼����Ⱦ����
��������
��1����ҵ�ϳ���ͨ��ֽ���������Al2O3���Ʊ���������ͬʱ�������������÷�Ӧ�Ļ�ѧ����ʽΪ��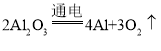 ��
��
�÷�Ӧ���ϡ�һ��ࡱ���ص㣬���ڷֽⷴӦ��
��2��������������Ͻ����������ڻ�����ղػ�档��ԭ�������ڿ��������������γ�һ�����ܵ���������Ĥ����������Ʒ����������ʴ��
���Ͻ���ϵ��ŵ㣺�ܶ�С��㡢Ӳ�ȴ�ȣ�
��3���ɼ�����ƥ��ʥ���ڰ���ƥ�˵ĺ�������ǰ���ð��澵������������ȼ���֡�����Ϊ���ֵ�ȼ���ṩ���㹻��������
���ȼ��ʱ����ѧ��ת��Ϊ���ܺ��ܣ�
��4�����麬̼Ԫ�أ������л��
����ȼ�����ɶ�����̼��ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��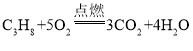 ��
��
����ȼ�Ϸ�������ɫ��������Ҫ��ԭ���ǣ�����ȼ��ֻ���ɶ�����̼��ˮ��������Ⱦ������