��Ŀ����
ij����������ʴÿ����ʧ����Լ���֮һ����Ҫ�ⶨ������ʴ����Ԫ�ص�������������ش��������⣺��1������100��14.6%��ϡ���ᣬ��Ҫ40%Ũ�����������g
��2����������Ʒ���飬��������ϡ����ʹ���ܽ�Ļ�ѧ����ʽ
��3����7.5�˸�����Ʒǡ����55�˵�14.6%ϡ������ȫ��Ӧ����Һ����62�˺�0.3������������Ʒ������������X������ʽ
��4������Ʒ����Ԫ�ص���������
��5����Ӧ����Һ���Ȼ���������������
��6����Ҫ����ij�ε�����Ҫ����30��֣�������������ʧ�����������Ƕ֣�
���𰸡���������1������ϡ��ǰ����Һ�����ʵ����������г���ʽ��
��2���˴������ķ�ӦΪ�������ᷢ���ķ�Ӧ��
��3����ѧ����ʽ��������������֮�䣨�������⣩�����ڹ̶��ı�����ϵ����֪��Ӧǰ��������������������غ㶨�ɿ��������������һ����д�������������ı���ʽ��
��4������������Һ�����ʵ�������������������������ٸ��� ��������������Ʒ������×100%���������Ʒ�е�����������������
��5����Һ�����ʵ���������=���ʵ�������Һ������×100%������Ϊ�Ȼ�������
��6����������ʯ�ֲ���������������г���ʽ��
����⣺��1�����������������Ϊx
100g×14.6%=x×40%
x=36.5g
��2���˴������ķ�ӦΪ��������ᡢ�������ᷢ���ķ�Ӧ����ѧ����ʽΪ��Fe2O3+6HCl=2FeCl3+3H2O��Fe+2HCl�TFeCl2+H2��
��3�����������������7.5g+55g-62g-0.3g=0.2g
��Ʒ����������Ϊx�������Ȼ�����������Ϊy
Fe+2HCl�TFeCl2+H2��
56 127 2
x y 0.2g

x=5.6g y=12.7g
��4��ԭ��Ʒ����Ԫ�ص���������= ×100%=89.6%
×100%=89.6%
��5����Ӧ��������Һ�����ʵ���������=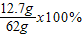 =20.5%
=20.5%
��6����ʧ�����������ǣ�30���×��1- ×100%��=7.6���
×100%��=7.6���
������������һ���ۺ��Եļ����⣬�Ƚ��ѣ����������Ҳ�϶࣬��Ҫע�����һ����Ӧ��������Һ�����ķ�������������������ʵ������ܺ�-���ɳ���������-�����������������������������Һ������ȴ������ļ��㷽���������ʵ�����+�ܼ���������
��2���˴������ķ�ӦΪ�������ᷢ���ķ�Ӧ��
��3����ѧ����ʽ��������������֮�䣨�������⣩�����ڹ̶��ı�����ϵ����֪��Ӧǰ��������������������غ㶨�ɿ��������������һ����д�������������ı���ʽ��
��4������������Һ�����ʵ�������������������������ٸ��� ��������������Ʒ������×100%���������Ʒ�е�����������������
��5����Һ�����ʵ���������=���ʵ�������Һ������×100%������Ϊ�Ȼ�������
��6����������ʯ�ֲ���������������г���ʽ��
����⣺��1�����������������Ϊx
100g×14.6%=x×40%
x=36.5g
��2���˴������ķ�ӦΪ��������ᡢ�������ᷢ���ķ�Ӧ����ѧ����ʽΪ��Fe2O3+6HCl=2FeCl3+3H2O��Fe+2HCl�TFeCl2+H2��
��3�����������������7.5g+55g-62g-0.3g=0.2g
��Ʒ����������Ϊx�������Ȼ�����������Ϊy
Fe+2HCl�TFeCl2+H2��
56 127 2
x y 0.2g

x=5.6g y=12.7g
��4��ԭ��Ʒ����Ԫ�ص���������=
 ×100%=89.6%
×100%=89.6%��5����Ӧ��������Һ�����ʵ���������=
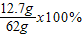 =20.5%
=20.5%��6����ʧ�����������ǣ�30���×��1-
 ×100%��=7.6���
×100%��=7.6���������������һ���ۺ��Եļ����⣬�Ƚ��ѣ����������Ҳ�϶࣬��Ҫע�����һ����Ӧ��������Һ�����ķ�������������������ʵ������ܺ�-���ɳ���������-�����������������������������Һ������ȴ������ļ��㷽���������ʵ�����+�ܼ���������

��ϰ��ϵ�д�
�����Ŀ
 ����������ʴÿ����ʧ����ԼΪ������20%��40%����Ҫ�ⶨ��ʴ�ĸ���������
����������ʴÿ����ʧ����ԼΪ������20%��40%����Ҫ�ⶨ��ʴ�ĸ��������� ����������ʴÿ����ʧ����ԼΪ������20%��40%����Ҫ�ⶨ��ʴ�ĸ���������
����������ʴÿ����ʧ����ԼΪ������20%��40%����Ҫ�ⶨ��ʴ�ĸ���������