��Ŀ����
�Ķ����ϣ��ش����⣺�������������������������ˮ���Ҹ�ˮ�������������SO2+H2O=H2SO3��������ܲ��ȶ������ֽ����ɶ��������ˮ��H2SO3=SO2+H2O������������Ư��ijЩ��ɫ���ʣ��罫��������ͨ��Ʒ����Һ�У���ʹƷ����Һ��ɫ�����������Ư���������������ܸ�ijЩ��ɫ���ʷ�Ӧ�����ɲ��ȶ�����ɫ���ʣ�������ɫ�������ֽ��ʹ��ɫ���ʻָ�ԭ������ɫ��ijѧ������С��������ͼ��ʾװ�ã��ֱ�������ʵ�飺
��1�����Թ���ע��ij��ɫ��Һ�������Թܣ���Һ��Ϊ��ɫ����ȴ��ָ���ɫ�������Һ������ ��Һ������ʱ����Һ����ɫ��Ϊ��ɫ��ԭ���� ��
��2�����Թ���ע��ij��ɫ��Һ�������Թܣ���Һ��ɫ��dz����ȴ��ָ���ɫ����ԭ��Һ������ ��Һ��
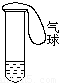
���𰸡���������1�����ݶ���������������������ɣ�����������ʹƷ����ɫ�����Ⱥ�����������ܸ�ijЩ��ɫ�������ɵ���ɫ�������ֽ⣮
��2�������ᡢ�������ָʾ���ı�ɫ������ƶ���Һ�����ͣ�������Ŀ�漰��ɫ�Ǻ�ɫ����ɫ���ʿɵ�֪��Һ�Ǽ�����ɫ��̪�Ļ��Һ��
����⣺��1����Ϊ����������ʹƷ����Һ��ɫ�����������Ư���������������ܸ�ijЩ��ɫ���ʷ�Ӧ�����ɲ��ȶ�����ɫ���ʣ�������ɫ�������ֽ��ʹ��ɫ���ʻָ�ԭ������ɫ��
�ʴ�Ϊ������SO2 ��Ʒ�죻SO2 �����ݳ���Ʒ����Һ�ָ���ɫ��
��2����Ϊ��ɫ��̪��Һ����ˮ�Ժ�ɫ�����ڰ�ˮ���лӷ��ԣ����Ե�����ʱ����ˮ�еİ������˶��������У���Һ��û�а������ˣ�����Һ�ɺ�ɫ�����ɫ��
�ʴ�Ϊ��ϡ��ˮ�ͷ�̪��
������������һ�����͵���Ϣ�⣻�ص㿼���˶�������������Լ����ָʾ����ʹ�ã�
��2�������ᡢ�������ָʾ���ı�ɫ������ƶ���Һ�����ͣ�������Ŀ�漰��ɫ�Ǻ�ɫ����ɫ���ʿɵ�֪��Һ�Ǽ�����ɫ��̪�Ļ��Һ��
����⣺��1����Ϊ����������ʹƷ����Һ��ɫ�����������Ư���������������ܸ�ijЩ��ɫ���ʷ�Ӧ�����ɲ��ȶ�����ɫ���ʣ�������ɫ�������ֽ��ʹ��ɫ���ʻָ�ԭ������ɫ��
�ʴ�Ϊ������SO2 ��Ʒ�죻SO2 �����ݳ���Ʒ����Һ�ָ���ɫ��
��2����Ϊ��ɫ��̪��Һ����ˮ�Ժ�ɫ�����ڰ�ˮ���лӷ��ԣ����Ե�����ʱ����ˮ�еİ������˶��������У���Һ��û�а������ˣ�����Һ�ɺ�ɫ�����ɫ��
�ʴ�Ϊ��ϡ��ˮ�ͷ�̪��
������������һ�����͵���Ϣ�⣻�ص㿼���˶�������������Լ����ָʾ����ʹ�ã�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 �Ķ����ϣ��ش����⣺
�Ķ����ϣ��ش����⣺