��Ŀ����
����Ŀ����ѧѧϰ�벻����ѧ�仯���仯ʵ�����ʼ���ת����
��1��Ca(OH)2����
����д��Ca(OH)2���ʵ�ԭ��______���û�ѧ����ʽ��ʾ����
��2�������о�Ca(OH)2���ʳ̶�
�������ʵ��ȷ��Ca(OH)2�ı��ʳ̶ȣ�д��������������______��
��3�������о�Ca(OH)2���ʳ̶�
������ϡ�����������300g��������Ϊ5%�����ᣬ��Ҫ��������Ϊ30%������______g��
���ƹ����У����ձ�������������Ͳ�⣬�����õ��IJ���������______������ȡ30%������ʱ���Ӷ����������Ƶ�������������______5%������ڡ�С�ڣ���
��ʵ��ⶨ����ȡ40.0g��Ʒ�����ձ��У���������ϡ�����ַ�Ӧ���ձ��������뷴Ӧʱ��Ĺ�ϵ�����ʾ��
��Ӧʱ��/min | 0 | t1 | t2 | t3 | t4 |
�ձ�������/g | 150.0 | 147.8 | 145.6 | 145.6 | 145.6 |
��������Ʒ��Ca(OH)2������������_____
���𰸡�CO2+Ca(OH)2=CaCO3��+H2O ȡ������Ʒ����ˮ�У��ټ����̪��Һ��������죬��������ȫ���ʣ������ɫ���������Ʋ��ֱ��� 50 ��ͷ�ι� С�� 75%
��������
������̼��������������̼��ƺ�ˮ��̼��ƺ�ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����Һϡ��ǰ�������������䡣
��1�����������Ʊ��ʵ�ԭ���ǿ����еĶ�����̼��������������̼��ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪCO2+Ca(OH)2=CaCO3��+H2O��
��2��������������Һ�Լ��ԣ���ʹ��̪��Һ��ɺ�ɫ��ȷ���������Ƶı��ʳ̶ȵIJ����������۵����鷽����ȡ������Ʒ����ˮ�У��ټ����̪��Һ��������죬��������ȫ���ʣ������ɫ���������Ʋ��ֱ��ʡ�
��3��������Ҫ��������Ϊ30%������Ϊx
![]()
![]()
����Ҫ��������Ϊ30%������50g��
���ƹ����У����ձ�������������Ͳ�⣬�����õ��IJ��������ǽ�ͷ�ιܣ�����ȡ30%������ʱ���Ӷ���������ƫ��ʵ����ȡ��Ũ����ƫ�٣������Ƶ�������������С��5%��
���ɱ��е����ݿ�֪��t2ʱ�ձ����������䣬��Ӧ��ȫ���������غ㶨�ɿ�֪����Ӧ�������������������������������ٵ�Ϊ���ɵ�����������������ɵĶ�����̼������Ϊ![]()
��̼��Ƶ�����Ϊy
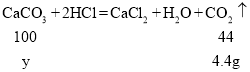
![]()
![]()
����Ʒ��Ca(OH)2������������![]()

 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�����Ŀ����ͼ��ʾ�����ձ�A��װ��10mLŨ��ˮ���ձ�B��װ��20mL����ˮ���������˼��η�̪��Һ����һ�����ձ���A��B����С�ձ�����һ��һ��ʱ���B�ձ��е���Һ��Ϊ��ɫ�����ڷ�̪��죬��ѧ̽��С��ͬѧ����������ֲ��룺( ��̪��Һ�Ƿ�̪��ˮ�Ļ����)
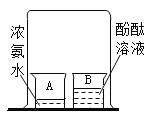
����һ����A�ձ���������ˮ����������ˮ�����˶�����B�ձ���ʹ��̪��죻
���������A�ձ��лӷ������������ְ������˶�����B�ձ���ʹ��̪��죻
����������A�ձ��лӷ������������ְ������˶�����B�ձ���ˮ����γɰ�ˮ��ʹ��̪��졣
(1)ͬѧ�Ǿ������ۺ�һ����Ϊ����һ�Dz����ܵģ�������_____��
(2)Ϊ��̽���������ֲ����Ƿ���ȷ��ͬѧ�����������ʵ�鷽�����������
ʵ����� | ʵ������ | ʵ����� |
��һ����ֽ�����̪��Һ�У�1min��ȡ����������ͨ�紦���ɣ��ü������������Ƭ | ||
������һƬ������ֽ�����ռ��������ļ���ƿ�� | ��ֽ����ɫ | ����____(ѡ������������������)ʹ��̪��죬�����___(ѡ������ȷ������������) |
����һƬ��ֽ�ϵμ�������ˮ���ٷ����ռ��������ļ���ƿ�� | ��ֽ���____ɫ | �����ܽ���ˮ���γɰ�ˮ����ˮ��ʹ��̪��죬������___(ѡ������ȷ������������) |
����Ŀ������ʵ�������ͼ���ϵ��Ӧ��ȷ���ǣ�������
ѡ�� | A | B | C | D |
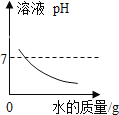
���� | NaOH��Һ�в��ϼ�ˮϡ�� | ˮ����������������� | �������ϡ�����м���������þ���� | �õ������������������Ĺ���������Һ��ȡ���� |
ͼ�� |
|
|
|
|
A.AB.BC.CD.D
����Ŀ�����г���(������Ϊ����)���Լ�ѡ����ȷ����( )
��� | ���� | ѡ���Լ� |
A | CaCl2��Һ(����) | ������Na2CO3��Һ |
B | NaOH��Һ(Na2CO3��Һ) | ������Ca(OH)2��Һ |
C | CuO(Cu) | ������ϡH2SO4 |
D | KCl��Һ(K2SO4��Һ) | ������Ba(NO3)2��Һ |
A.AB.BC.CD.D