��Ŀ����
��2013?���ң�����ơ���һ�ֿ�����ϸ�����Ⱥܸߵ�̼��Ʒ�ĩ���й㷺����;��������������Ƭ�����εȣ�����ij��Ƴ��õ��طḻ��ʯ��ʯ��ͨ�����������ơ���ơ���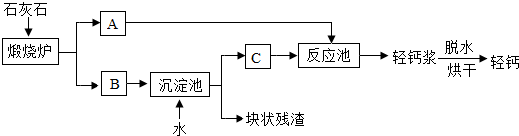
��1��̼����и�Ԫ�ص�����������
��2��ʯ��ʯ������ת��ΪA��B���÷�Ӧ����
��3���������еõ��Ŀ�״�������ܺ���δ����ʯ��ʯ��������Ա��������м��飬�۲쵽
��4������������Ա���������̼���ƴ��������̼���������Ʒ�Ӧ��������̼��Ƶ�ͬʱ���ɵõ��������ƣ�һ����Ҫ�ļ����Ӧ��ԭ����Na2CO3+Ca��OH��2=CaCO3��+2NaOH����ͨ���������������ַ�������50t̼���ʱ���ܵõ��������Ƶ������Ƕ��٣�

��1��̼����и�Ԫ�ص�����������
40
40
%��ʳ��������̼��������ڷ�ֹ����ȱ���������������֢
��������֢
�����������֢����ƶѪ����������2��ʯ��ʯ������ת��ΪA��B���÷�Ӧ����
�ֽⷴӦ
�ֽⷴӦ
���������Ӧ���ͣ�����3���������еõ��Ŀ�״�������ܺ���δ����ʯ��ʯ��������Ա��������м��飬�۲쵽
�����ݲ���
�����ݲ���
��֤�������к���ʯ��ʯ����4������������Ա���������̼���ƴ��������̼���������Ʒ�Ӧ��������̼��Ƶ�ͬʱ���ɵõ��������ƣ�һ����Ҫ�ļ����Ӧ��ԭ����Na2CO3+Ca��OH��2=CaCO3��+2NaOH����ͨ���������������ַ�������50t̼���ʱ���ܵõ��������Ƶ������Ƕ��٣�
��������1�����ݻ�������ijԪ�ص�����������ʽ�����Ԫ�ص��������������ݸ�Ԫ�ض���������÷�����
��2������̼����ڸ����²��������ƺ�ˮ������Ӧ���ͣ�
��3������̼��ƺ����ᷴӦ����������̼�����������
��4�����ݻ�ѧ����ʽ��̼��ƺ��������ƵĹ�ϵ�б������㣮
��2������̼����ڸ����²��������ƺ�ˮ������Ӧ���ͣ�
��3������̼��ƺ����ᷴӦ����������̼�����������
��4�����ݻ�ѧ����ʽ��̼��ƺ��������ƵĹ�ϵ�б������㣮
����⣺��1��̼����и�Ԫ�ص���������Ϊ��
��100%=40%��
��Ԫ�������ڹ�������������ʳ��������̼��������ڷ�ֹ����ȱ������Ĺ�������֢����ƶѪ��ȱ������ģ�
��2��̼����ڸ����²��������ƺ�ˮ����һ�����������������ʵķֽⷴӦ��
��3��̼��ƺ����ᷴӦ����������̼���ʻ�۲쵽�����ݲ�����
��4���⣺��õ��������Ƶ�����Ϊ x
Na2CO3+Ca��OH��2=CaCO3��+2NaOH
100 80
50t x
=
x=40t
���ܵõ��������Ƶ�����Ϊ40t��
�ʴ�Ϊ����1��40 ��������֢��
��2���ֽⷴӦ��
��3����������
��4���⣺��õ��������Ƶ�����Ϊ x
Na2CO3+Ca��OH��2=CaCO3��+2NaOH
100 80
50t x
=
x=40t
���ܵõ��������Ƶ�����Ϊ40t��
| 40 |
| 100 |
��Ԫ�������ڹ�������������ʳ��������̼��������ڷ�ֹ����ȱ������Ĺ�������֢����ƶѪ��ȱ������ģ�
��2��̼����ڸ����²��������ƺ�ˮ����һ�����������������ʵķֽⷴӦ��
��3��̼��ƺ����ᷴӦ����������̼���ʻ�۲쵽�����ݲ�����
��4���⣺��õ��������Ƶ�����Ϊ x
Na2CO3+Ca��OH��2=CaCO3��+2NaOH
100 80
50t x
| 100 |
| 50t |
| 80 |
| x |
x=40t
���ܵõ��������Ƶ�����Ϊ40t��
�ʴ�Ϊ����1��40 ��������֢��
��2���ֽⷴӦ��
��3����������
��4���⣺��õ��������Ƶ�����Ϊ x
Na2CO3+Ca��OH��2=CaCO3��+2NaOH
100 80
50t x
| 100 |
| 50t |
| 80 |
| x |
x=40t
���ܵõ��������Ƶ�����Ϊ40t��
���������⿼������̼����йص�Ԫ�����������ļ��㡢��Ӧ���͵ķ��������鼰���ݻ�ѧ����ʽ�ļ��㣬�ѶȲ���������ص�֪ʶ��һ�������ɣ�

��ϰ��ϵ�д�
�����Ŀ


 ��2013?���ң���ͼΪNaCl��KNO3���ܽ�����ߣ�����˵����ȷ���ǣ�������
��2013?���ң���ͼΪNaCl��KNO3���ܽ�����ߣ�����˵����ȷ���ǣ�������