��Ŀ����
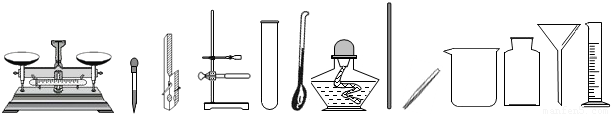
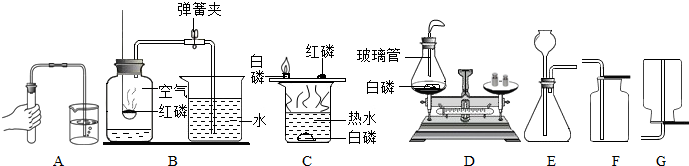
��������ͼʾ�dz��л�ѧ�γ��г��õ�������ʵ��װ�ã��밴Ҫ��ش�1��-��7���е����⣺
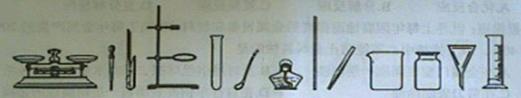
��1�����ڶ��������ģ���дһ���������ƣ��磺������ƽ��______�ȣ�
��2������ȡ��ҩƷ����������дһ���������ƣ��磺���ӡ�______�ȣ�
��3��������������������дһ���������ƣ��磺�Թܡ�______�ȣ�
��4�����ڹ��˵������ǣ���д������Ҫ���������ƣ�______��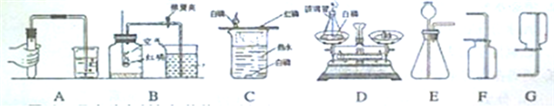
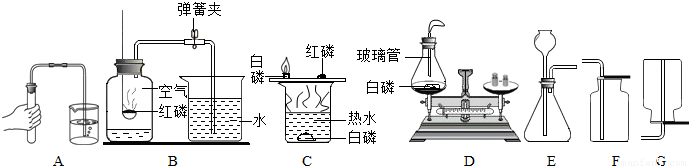
��5��ͼʾA��ʵ�����Ʊ�����ǰ�����IJ��裬�����ֱ���Ϊ______��
��6��B��C��D����������ȼ���йص�ʵ�飬��ʵ��Ŀ�ķֱ��ǣ��磺B-�ⶨ�����������ĺ�����������C��D����ѡһ������______��
��7����װ��E����ȡ�������壬����E+F��Ͽ���ȡ�������ǣ�д��ѧʽ��______������E+G��Ͽ���ȡ����Ļ�ѧ����ʽ��______��
�⣺��1�����ڶ����������п̶ȵĻ�����Ͳ��
��2������ȡ��ҩƷ����������ȡ�÷�ĩ״ҩƷ��ҩ�ס��μ�����Һ��Ľ�ͷ�ιܣ�
��3�����������Ļ����ձ�������ƿ��
��4��������Ҫ�������У�����̨��©�����ձ�����������
��5���ò����Ǽ��װ�õ������ԣ������ܿ��Ƿ�������ð����
��6��C��ͨ��ˮ�еİ���ͭƬ�ϵİ��Ա�˵��ȼ����Ҫ�߱��������Ӵ���ͭƬ�ϵİ��ͺ��Ա�˵��ȼ����Ҫ�߱��¶ȴﵽ��ȼ����Ż�㣬����̽��ȼ�յ�������
D����������ƽ�����ܱ�������Ӧǰ��������仯����֤�����غ㶨�ɣ�
��7��E+F����ʺϹ�Һ�����͵�������ȡ�����ܶȴ��ڿ�������������������������̼��E+G����ʺϹ�Һ�����͵�������ȡ�����ܶ�С�ڿ�������������������������IJ�����Ҫ���ý�������ķ�Ӧ�������ǣ�Zn+2HCl�TZnCl2+H2����Zn+H2SO4�TZnSO4+H2����
�ʴ�Ϊ����1����Ͳ����2��ҩ�ף���ͷ�ιܣ���
��3���ձ�������ƿ����
��4������̨��©�����ձ�����������
��5�����װ�õ������ԣ�
��6��C-̽��ȼ�յ�����������D-��֤�����غ㶨�ɣ�
��7��O2��CO2��Zn+2HCl�TZnCl2+H2����Zn+H2SO4�TZnSO4+H2��
��������1�����ݶ��������п̶ȿ��Զ�����ֵѡ��������
��2��ȡ��ҩƷ����������ȡ�ÿ�״�ͷ�ĩ״��ҩƷ��
��3����������������ֻҪ��ʢװҩƷ���ɣ�
��4�����ݹ��˲�������������ѡ��
��5�����ݲ���д����˫�ּ��ȿ����ܿ��Ƿ�������ݣ�
��6������ҩƷ������������C��ͨ�����ͺ��ĶԱ�̽��ȼ�յ�����������D����������ƽ�����ܱ�������Ӧǰ��������仯����֤�����غ㶨�ɣ�
��7�����ݷ���װ�ú��ռ��ķ���ѡ����ʵ����ʣ�E+F�ʺϹ�Һ�����͵�������ȡ�����ܶȴ��ڿ�����E+G����ʺϹ�Һ�����͵�������ȡ�����ܶ�С�ڿ��������壮
���������⿼���˳������������ƺ���;���Լ�����װ�õ���;�����ڻ�ѧ����֪ʶ�Ŀ��飬��ѧϰ��ѧ�Ļ�����Ҫ��ѧ��Ҫ�������գ�
��2������ȡ��ҩƷ����������ȡ�÷�ĩ״ҩƷ��ҩ�ס��μ�����Һ��Ľ�ͷ�ιܣ�
��3�����������Ļ����ձ�������ƿ��
��4��������Ҫ�������У�����̨��©�����ձ�����������
��5���ò����Ǽ��װ�õ������ԣ������ܿ��Ƿ�������ð����
��6��C��ͨ��ˮ�еİ���ͭƬ�ϵİ��Ա�˵��ȼ����Ҫ�߱��������Ӵ���ͭƬ�ϵİ��ͺ��Ա�˵��ȼ����Ҫ�߱��¶ȴﵽ��ȼ����Ż�㣬����̽��ȼ�յ�������
D����������ƽ�����ܱ�������Ӧǰ��������仯����֤�����غ㶨�ɣ�
��7��E+F����ʺϹ�Һ�����͵�������ȡ�����ܶȴ��ڿ�������������������������̼��E+G����ʺϹ�Һ�����͵�������ȡ�����ܶ�С�ڿ�������������������������IJ�����Ҫ���ý�������ķ�Ӧ�������ǣ�Zn+2HCl�TZnCl2+H2����Zn+H2SO4�TZnSO4+H2����
�ʴ�Ϊ����1����Ͳ����2��ҩ�ף���ͷ�ιܣ���
��3���ձ�������ƿ����
��4������̨��©�����ձ�����������
��5�����װ�õ������ԣ�
��6��C-̽��ȼ�յ�����������D-��֤�����غ㶨�ɣ�
��7��O2��CO2��Zn+2HCl�TZnCl2+H2����Zn+H2SO4�TZnSO4+H2��
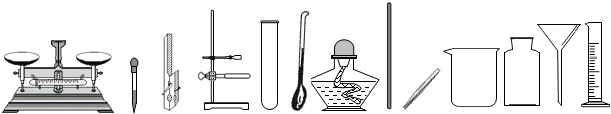
��������1�����ݶ��������п̶ȿ��Զ�����ֵѡ��������
��2��ȡ��ҩƷ����������ȡ�ÿ�״�ͷ�ĩ״��ҩƷ��
��3����������������ֻҪ��ʢװҩƷ���ɣ�
��4�����ݹ��˲�������������ѡ��
��5�����ݲ���д����˫�ּ��ȿ����ܿ��Ƿ�������ݣ�
��6������ҩƷ������������C��ͨ�����ͺ��ĶԱ�̽��ȼ�յ�����������D����������ƽ�����ܱ�������Ӧǰ��������仯����֤�����غ㶨�ɣ�
��7�����ݷ���װ�ú��ռ��ķ���ѡ����ʵ����ʣ�E+F�ʺϹ�Һ�����͵�������ȡ�����ܶȴ��ڿ�����E+G����ʺϹ�Һ�����͵�������ȡ�����ܶ�С�ڿ��������壮
���������⿼���˳������������ƺ���;���Լ�����װ�õ���;�����ڻ�ѧ����֪ʶ�Ŀ��飬��ѧϰ��ѧ�Ļ�����Ҫ��ѧ��Ҫ�������գ�

��ϰ��ϵ�д�
 �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д� ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
�����Ŀ

 ��ʵ�飬��ʵ��Ŀ�ķֱ��ǣ��磺B-�ⶨ�����������ĺ�����������C��D����ѡһ������ ��
��ʵ�飬��ʵ��Ŀ�ķֱ��ǣ��磺B-�ⶨ�����������ĺ�����������C��D����ѡһ������ ��
 �����ڶ��������ģ���дһ���������ƣ��磺������ƽ�� �ȣ�
�����ڶ��������ģ���дһ���������ƣ��磺������ƽ�� �ȣ� �� ͼʾA��ʵ�����Ʊ�����Ǯ�����IJ��裬�����ֱ���Ϊ ��
�� ͼʾA��ʵ�����Ʊ�����Ǯ�����IJ��裬�����ֱ���Ϊ ��