��Ŀ����
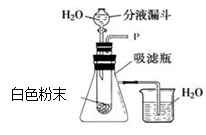
ij��ѧ��ȤС�����������ͼ��ʾʵ��װ�á�

�� ��Һ©�����������е�Һ�岻�����£����ܵ�ԭ���� ��
�� ��������������ʹ���е���ɫҺ�����Թ��еĹ���Ӵ���Ӧ���ɹ۲쵽�����ʹ�������ֱ�д��һ������ͼ�����������Ҫ��Ļ�ѧ����ʽ��
�٣��ֽⷴӦ ��
�ڣ����Ϸ�Ӧ�� �������ʹ��ԭ�� ��
�� ����Һ©����Һ��Ϊϡ���ᣬ����������Щ���������������ᷢ����ѧ��Ӧ����ʹ�����ʹ���� ������ţ��� ��Na2CO3 ��Zn ��Cu ��NaCl

�� ��Һ©�����������е�Һ�岻�����£����ܵ�ԭ���� ��
�� ��������������ʹ���е���ɫҺ�����Թ��еĹ���Ӵ���Ӧ���ɹ۲쵽�����ʹ�������ֱ�д��һ������ͼ�����������Ҫ��Ļ�ѧ����ʽ��
�٣��ֽⷴӦ ��
�ڣ����Ϸ�Ӧ�� �������ʹ��ԭ�� ��
�� ����Һ©����Һ��Ϊϡ���ᣬ����������Щ���������������ᷢ����ѧ��Ӧ����ʹ�����ʹ���� ������ţ��� ��Na2CO3 ��Zn ��Cu ��NaCl
| A���٢� | B���٢ڢ� | C���٢ڢ� | D���٢ڢۢ� |
���� ��Һ©��ƿ��δ��
�� �� 2H2O2
 2H2O+O2�� �� CaO+H2O ==Ca(OH)2��
2H2O+O2�� �� CaO+H2O ==Ca(OH)2��
��������ˮ��Ӧ���ȣ�ƿ������������͡�
�� A ������ʽ2�֣�����ÿ��1�֣�
�� �� 2H2O2

 2H2O+O2�� �� CaO+H2O ==Ca(OH)2��
2H2O+O2�� �� CaO+H2O ==Ca(OH)2����������ˮ��Ӧ���ȣ�ƿ������������͡�
�� A ������ʽ2�֣�����ÿ��1�֣�
��1����Һ©����Һ�岻�ܵ��£���ѹǿ�й�ϵ��
��2�������������ͣ���֪Һ�������ܷ�����ѧ��Ӧ���������壬Ҳ�����ǹ�����������ˮ�ų��������ȣ��Թ��еĿ������͵��£�
�Ǹ����ܺ���������������н��
��2�������������ͣ���֪Һ�������ܷ�����ѧ��Ӧ���������壬Ҳ�����ǹ�����������ˮ�ų��������ȣ��Թ��еĿ������͵��£�
�Ǹ����ܺ���������������н��

��ϰ��ϵ�д�
�����Ŀ