��Ŀ����
��ѧ�ڷ��δ�����Ⱦ��ˮ��Ⱦ�ȷ��淢������Ҫ���ã�ʹ����ػ�ѧ�������պͲ�Ʒ���Ż����Ѻõķ���չ��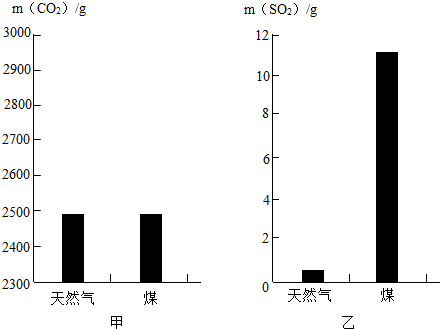
��1��Ϊ��Դͷ������Ⱦ��������������Ҫѡ��Ի���Ӱ��С��ȼ�ϣ���֪���ȼ��1kg��Ȼ����ú��������CO2��SO2�����������ͼ��ʾ����______ȼ�ղ�����������������꣮
��2��ij�������糧Ϊ�˷�ֹ������Ⱦ���轫�����еĶ���������д������䷢������Ҫ��ӦΪ2CaCO3+2SO2+O2=2CaSO4+2CO2����100g�����к���1.6g������������1000g���ַ����躬̼���80%��ʯ��ʯ______g��
��3��ij����������10L/s����/�룩�ų�������9.8%�Ĺ�ҵ��ˮ������÷�ˮ�����뺬��������2%�Ĺ�ҵ��ˮ�������ᷴӦ�����ų���Ҫʹ������ķ�ˮ�����ԣ�����������2%�Ĺ�ҵ��ˮ������ӦΪ______L/s�����������ˮ�ͺ����ˮ���ܶȾ�����Ϊ1g/cm3����

��1��Ϊ��Դͷ������Ⱦ��������������Ҫѡ��Ի���Ӱ��С��ȼ�ϣ���֪���ȼ��1kg��Ȼ����ú��������CO2��SO2�����������ͼ��ʾ����______ȼ�ղ�����������������꣮
��2��ij�������糧Ϊ�˷�ֹ������Ⱦ���轫�����еĶ���������д������䷢������Ҫ��ӦΪ2CaCO3+2SO2+O2=2CaSO4+2CO2����100g�����к���1.6g������������1000g���ַ����躬̼���80%��ʯ��ʯ______g��
��3��ij����������10L/s����/�룩�ų�������9.8%�Ĺ�ҵ��ˮ������÷�ˮ�����뺬��������2%�Ĺ�ҵ��ˮ�������ᷴӦ�����ų���Ҫʹ������ķ�ˮ�����ԣ�����������2%�Ĺ�ҵ��ˮ������ӦΪ______L/s�����������ˮ�ͺ����ˮ���ܶȾ�����Ϊ1g/cm3����
��1����ͼ�п��Կ�����ȼ��1kg��Ȼ����ú�������Ķ�����̼�����൱����ȼ��1kg��Ȼ����ú�������Ķ����������ܴ�ȼ��1kgúʱ�����Ķ��������ȼ��1kg��Ȼ��ʱ��ܶ࣬����ȼ��úʱ������������������꣮
���ú��
��2������̼��Ƶ�����ΪX��
1000g���ַ����ж�������������ǣ�1.6g��
=16g��
2CaCO3+2SO2+O2=2CaSO4+2CO2��
200 128
X 16g
=
X=25g��
��̼���80%��ʯ��ʯ������Ϊ��
25g��80%=31.25g��
���31.25��
��3����ÿ���ų����������Ƶ�����ΪX��
1g/cm3=1000g/L
ÿ���ų������������Ϊ��10L/s��1s��1000g/L��9.8%=980g��
2NaOH+H2SO4�TNa2SO4+2H2O
80 98
X 980g
=
��
X=800g��
ÿ���ų�������������Һ������Ϊ��
800g��2%=40000g��
����������2%�Ĺ�ҵ��ˮ������ӦΪ��
40000g��1000g/L��1s=40L/s��
���40��
���ú��
��2������̼��Ƶ�����ΪX��
1000g���ַ����ж�������������ǣ�1.6g��
| 1000g |
| 100g |
2CaCO3+2SO2+O2=2CaSO4+2CO2��
200 128
X 16g
| 200 |
| 128 |
| X |
| 16g |
X=25g��
��̼���80%��ʯ��ʯ������Ϊ��
25g��80%=31.25g��
���31.25��
��3����ÿ���ų����������Ƶ�����ΪX��
1g/cm3=1000g/L
ÿ���ų������������Ϊ��10L/s��1s��1000g/L��9.8%=980g��
2NaOH+H2SO4�TNa2SO4+2H2O
80 98
X 980g
| 80 |
| 98 |
| X |
| 980g |
X=800g��
ÿ���ų�������������Һ������Ϊ��
800g��2%=40000g��
����������2%�Ĺ�ҵ��ˮ������ӦΪ��
40000g��1000g/L��1s=40L/s��
���40��

��ϰ��ϵ�д�
�����Ŀ

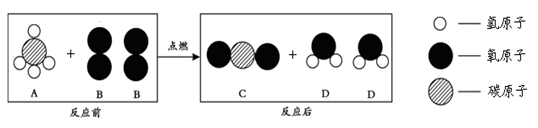
 CO2+ 2H2O
CO2+ 2H2O