��Ŀ����
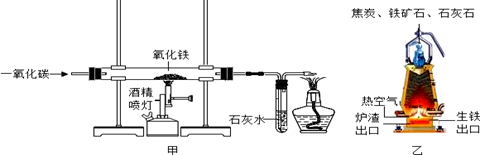
��5�֣�ij��ѧС����ʵ������CO��Fe2O3���ܱ������и���������ǿ��ģ���������̣�һ��ʱ����ռ����������ݣ�
��1��Ԥ�ڿ�����ʵ������Ӧ���� ��
��2��ʵ������п�����ʵ��������Ԥ�ڵ�������������������Ͽ�֪��CO��ԭFe2O3ʱ��һ�������¿�������������������FeO��Fe3O4������Ҳ��Ϊ��ɫ���壬�����Ļ�ѧ��Ӧ����ʽ�ֱ�ΪFe2O3+CO 2FeO+CO2�� .
2FeO+CO2�� .
��2������b��ֵΪ ������M�Ļ�ѧʽΪ ��
| ���� | CO | Fe2O3 | Fe | M | CO2 |
| ��Ӧǰ������g�� | 2.8 | 16 | 0 | 0 | 0 |
| ��Ӧ��������g�� | 0 | 0 | 0 | a | b |
��2��ʵ������п�����ʵ��������Ԥ�ڵ�������������������Ͽ�֪��CO��ԭFe2O3ʱ��һ�������¿�������������������FeO��Fe3O4������Ҳ��Ϊ��ɫ���壬�����Ļ�ѧ��Ӧ����ʽ�ֱ�ΪFe2O3+CO
 2FeO+CO2�� .
2FeO+CO2�� .��2������b��ֵΪ ������M�Ļ�ѧʽΪ ��
��1����ɫ�����ɺ�ɫ���� ��2��3Fe2O3+CO ����2Fe3O4+CO2
��3�� 4.4 FeO
��3�� 4.4 FeO
�����������1����Ӧ�õ����ۣ��Ǻ�ɫ������Ԥ�ڿ�����ʵ������Ӧ���ǣ���ɫ�����ɺ�ɫ����
��2��CO��ԭFe2O3ʱ��һ�������¿�������������������Fe3O4�������Ļ�ѧ��Ӧ����ʽΪ��3Fe2O3+CO 2Fe3O4+CO2
��3�����������غ㶨�ɣ�Ԫ�ص��������䣬����2.8g��12/28��100%=b��12/44��100%�������b=4.4g��
ͬ�����������غ㶨�ɣ�����M������a=2.8g+16g-4.4g=14.4g���ٸ�����Ԫ�ص������������Ƴ�����M�Ļ�ѧʽΪFeO

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ