��Ŀ����
��ʵ����������У�С��ͬѧ�鵽����Ŀ�ǡ�������̼����ȡ. �ռ�����������С��ͬѧ�鵽����Ŀ�ǡ���������ȡ. �ռ�����������
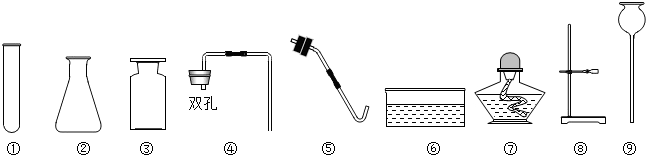
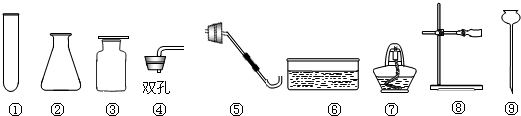
��1��Ҫ��ȡ������̼��С��ͬѧӦ�ô�����ҩƷ��ѡ��_________��__________��Ӧ��
��2��ѡ������ҩƷ��������____________(�����)
�ٲ�����Ũ���ᣬ��ΪŨ�����ӷ���ʹ�Ȼ���������������̼�����С�
��һ�㲻ѡ��̼���Ʒ�ĩ����Ϊ�����ᷴӦ�ٶ�̫�죬�������ռ���
�ۿ���ľ̿��������Ӧ����Ϊ�������ɶ�����̼��
�ܿ���ϡ������ʯ��ˮʯ��Ӧ����Ϊ��Ӧ�ٶ����У�����Ҫ�ߣ�������﴿���������ռ���
ʵ��̨���ṩ�����¼���װ�ã���ش�
��2��ѡ������ҩƷ��������____________(�����)
�ٲ�����Ũ���ᣬ��ΪŨ�����ӷ���ʹ�Ȼ���������������̼�����С�
��һ�㲻ѡ��̼���Ʒ�ĩ����Ϊ�����ᷴӦ�ٶ�̫�죬�������ռ���
�ۿ���ľ̿��������Ӧ����Ϊ�������ɶ�����̼��
�ܿ���ϡ������ʯ��ˮʯ��Ӧ����Ϊ��Ӧ�ٶ����У�����Ҫ�ߣ�������﴿���������ռ���
ʵ��̨���ṩ�����¼���װ�ã���ش�
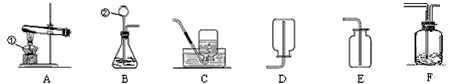
��3��д����ͼ�д�������������ƣ���__________����__________��
��4��С�к�С��ͬѧ������ͬһ��װ������ȡ������̼����������ѡ��ķ���װ�ú��ռ�װ��Ϊ__________������ĸ������д������һ����ѧ����ʽ________________________________�������Ƶõ�������������ˮ�������������Ƚ�����ͨ��Fװ�ã���ʱFװ����ʢװ���Լ���_________��
��5����Ȼ������Ҫ�ɷּ�����һ����ɫ��ζ���ܶȱȿ���С����������ˮ�����壬ʵ�����ü�����ˮ�����ƹ�����������ƹ�������ȡ������������Ϣ��֪��ʵ������ȡ�����ѡ�õ������__________������ĸ����
��4��С�к�С��ͬѧ������ͬһ��װ������ȡ������̼����������ѡ��ķ���װ�ú��ռ�װ��Ϊ__________������ĸ������д������һ����ѧ����ʽ________________________________�������Ƶõ�������������ˮ�������������Ƚ�����ͨ��Fװ�ã���ʱFװ����ʢװ���Լ���_________��
��5����Ȼ������Ҫ�ɷּ�����һ����ɫ��ζ���ܶȱȿ���С����������ˮ�����壬ʵ�����ü�����ˮ�����ƹ�����������ƹ�������ȡ������������Ϣ��֪��ʵ������ȡ�����ѡ�õ������__________������ĸ����
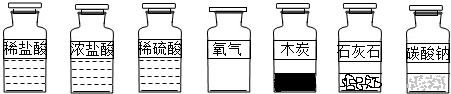
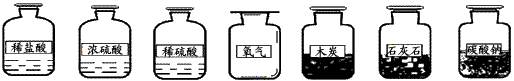
��1��ϡ���ʯ��ʯ
��2���٢ڢ�
��3���ƾ��ƣ�����©��
��4��BE�� CaCO3+2HCl===CaCl2+H2O+CO2������2H2O2 2H2O+O2�� ��Ũ����
2H2O+O2�� ��Ũ����
��5��AC��AD
��2���٢ڢ�
��3���ƾ��ƣ�����©��
��4��BE�� CaCO3+2HCl===CaCl2+H2O+CO2������2H2O2
 2H2O+O2�� ��Ũ����
2H2O+O2�� ��Ũ���� ��5��AC��AD

��ϰ��ϵ�д�
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�
�����Ŀ