��Ŀ����
 ��2011?���ݣ�ijѧϰС���������������о���
��2011?���ݣ�ijѧϰС���������������о�����1����ͼ�������ٵ�����Ϊ
��Һ©��
��Һ©��
��������Ӧ�Ļ�ѧ����ʽΪZn+H2SO4=ZnSO4+H2��
Zn+H2SO4=ZnSO4+H2��
����װ��Ҳ������ʵ������������̼
������̼
���壨��дһ�֣�����ѧ����ʽΪCaCO3+2HCl=CaCl2+H2O+CO2��
CaCO3+2HCl=CaCl2+H2O+CO2��
����2����֪��ͬ�¶��£��������������Խ����Һ������Խǿ��������18%��ϡ�����pH
��
��
2% ��ϡ���ᣨ���������������=����������ϡ����������²�������Һ��pH��ѧ�仯�����ߵ���BC
BC
������ĸ����A��������ˮ B������������ C���������������� D��������Ũ����
��3���������ձ��зֱ�ע��Ũ���ᣨ������������Ϊ98%���ܶ�Ϊ1.84g/m3����ϡ���ᣨŨ������ˮ�������1��1����10ml����������¼��ͬʱ�䣨h������������Һ��ˮ��������g�����ݼ��±���
| ʱ��/h | 1 | 1.5 | 4 | 8 | 12 | 24 | 48 | 60 | |
| ��ˮ����/g | Ũ���� | 1.6 | 2.2 | 6.2 | 10.3 | 14.0 | 20.9 | 29.2 | 32.1 |
| ϡ���� | 1.2 | 1.5 | 3.5 | 5.9 | 8.1 | 12.9 | 19.5 | 21.0 | |
| v��1.84g/cm3��98% |
| v��1.84g/cm3+v��1g/cm3 |
| v��1.84g/cm3��98% |
| v��1.84g/cm3+v��1g/cm3 |
ƫС
ƫС
���ƫ����ƫС�����䡱�����ɱ���ʵ�����ݿ�֪��
a���й�������ˮ�Ե�˵������ȷ����
B
B
������ĸ����A��Ũ��ϡ���ᶼ����ˮ�� B��ֻ��Ũ��������ˮ�� C��Ũ������ˮ�Խ�ϡ����ǿ
b��ʵ���ұ���ϡ����ʱҪ
�ܷ�
�ܷ�
��ţ��ڸ��������Ӧѡ��Ũ
Ũ
��������������Ũ����ϡ��������������1�����ݳ���������ͼ�κ�����д�������������ƣ����ݷ�Ӧ����������д����Ӧ�Ļ�ѧ����ʽ��������ȡװ�õ�ԭ��������Ӧ���״̬�ͷ�Ӧ������ѡ��ҩƷ��ȡ���壻
��2��������Һ�������������֮��Ĺ�ϵ������
��3���ٸ����������������ļ��㹫ʽ���������Ϊ1��1ϡ�������������������������ȡ���Ӷ���ʱʵ����ȡ��Ũ��������������õ�ϡ������������������ı仯��
��a���ݷ������е����ݣ������������ˮ�ԣ�b����Ũ�������ˮ�Է�����
��2��������Һ�������������֮��Ĺ�ϵ������
��3���ٸ����������������ļ��㹫ʽ���������Ϊ1��1ϡ�������������������������ȡ���Ӷ���ʱʵ����ȡ��Ũ��������������õ�ϡ������������������ı仯��
��a���ݷ������е����ݣ������������ˮ�ԣ�b����Ũ�������ˮ�Է�����
����⣨1��ͼ�������ٵ�����Ϊ��Һ©����п��ϡ���ᷴӦ����������п����������Ӧ�ķ���ʽ�ǣ�Zn+H2SO4=ZnSO4+H2��������ȡ�����װ�ÿ�֪����Ӧ���״̬�����Һ�壬��Ӧ��������Ҫ���ȣ���������װ�ÿ�ѡ��ʯ��ʯ�����ᷴӦ��ȡ������̼����Ӧ�ķ���ʽ�ǣ�CaCO3+2HCl=CaCl2+H2O+CO2����
��2����Ϊ��ͬ�¶��£��������������Խ����Һ������Խǿ����Һ��pHԽС�����Գ�����18%��ϡ�����pH��2% ��ϡ�����ϡ�����м�������ʱ���������ᷴӦ������ת��������Һ��pHҪ������ϡ�����м�����������ʱ���������غ����ᷴӦ����Һ��pHҪ�������ԣ���Һ��pH��ѧ�仯�����ߵ��� BC��
��3����Ҫ���������Ϊ1��1ϡ���ᣬ��ȡ������������Ϊ98%��Ũ��������Ϊv��ȡˮ�����ҲΪv���������Ϊ1��1ϡ������������������������ʽΪ��
��100%������ȡʱŨ����ʱ�Ǹ��Ӷ�����ʵ����ȡ��Ũ�������ˣ������õ�ϡ�������������ƫС��
��a�ɱ��е����ݿ�֪��Ũ��ϡ���ᶼ����ˮ�ԣ�Ũ�������ˮ�Ա�ϡ�������ˮ��ǿ��
b����Ũ��������ˮ�ԣ����ԣ�Ũ�����ڱ���ʱҪ�ܷⱣ�棬������ijЩ����ĸ������
�ʴ�Ϊ��
��1����Һ©����Zn+H2SO4=ZnSO4+H2����������̼��CaCO3+2HCl=CaCl2+H2O+CO2����
��2������B C��
��3����Ϊ
��100%��ƫС����a B��b�ܷ⣬Ũ��
��2����Ϊ��ͬ�¶��£��������������Խ����Һ������Խǿ����Һ��pHԽС�����Գ�����18%��ϡ�����pH��2% ��ϡ�����ϡ�����м�������ʱ���������ᷴӦ������ת��������Һ��pHҪ������ϡ�����м�����������ʱ���������غ����ᷴӦ����Һ��pHҪ�������ԣ���Һ��pH��ѧ�仯�����ߵ��� BC��
��3����Ҫ���������Ϊ1��1ϡ���ᣬ��ȡ������������Ϊ98%��Ũ��������Ϊv��ȡˮ�����ҲΪv���������Ϊ1��1ϡ������������������������ʽΪ��
| v��1.84g/cm3��98% |
| v��1.84g/cm3+v��1g/cm3 |
��a�ɱ��е����ݿ�֪��Ũ��ϡ���ᶼ����ˮ�ԣ�Ũ�������ˮ�Ա�ϡ�������ˮ��ǿ��
b����Ũ��������ˮ�ԣ����ԣ�Ũ�����ڱ���ʱҪ�ܷⱣ�棬������ijЩ����ĸ������
�ʴ�Ϊ��
��1����Һ©����Zn+H2SO4=ZnSO4+H2����������̼��CaCO3+2HCl=CaCl2+H2O+CO2����
��2������B C��
��3����Ϊ
| v��1.84g/cm3��98% |
| v��1.84g/cm3+v��1g/cm3 |
������������Ҫ�������й������֪ʶ��Ҫ�����������Ŀ����Ҫ������������Ļ�ѧ���ʡ���;����Ӧ����ͻ�ѧ����ʽ���Լ���֮��ص�֪ʶ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

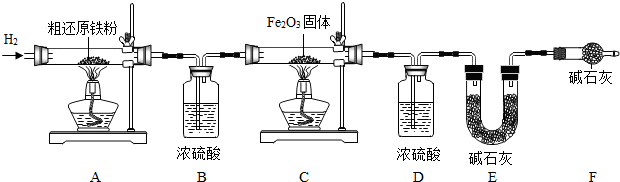
 ��2011?���ݣ���һ������ ��ı�ǩ��ͼ��
��2011?���ݣ���һ������ ��ı�ǩ��ͼ��