��Ŀ����
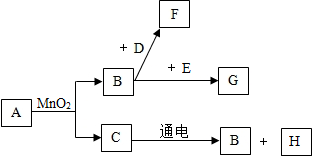
A��G�dz��г�����7�����ʣ���������ͼ��ʾ��ת����ϵ�����ֲ�������ȥ������֪A��C����ɫҺ�壬B��F����ɫ���壬���д����ŷ�F����������ЧӦ��D�Ǻ�ɫ���壬G���Ϻ�ɫ���壮
��1����д���������ʵĻ�ѧ���ţ�A
��2����˵��B������������������;
��3����д�����з�Ӧ�����ֱ���ʽ������������Ӧ���ͣ�����Ϸ�Ӧ��ֽⷴӦ��B+D��F

��1����д���������ʵĻ�ѧ���ţ�A
H2O2
H2O2
��GKMnO4
KMnO4
��FCO2
CO2
����2����˵��B������������������;
������ȼ��
������ȼ��
�������һ�ּ��ɵ÷֣� ��̬F���ʱ���Ϊ�ɱ�
�ɱ�
����3����д�����з�Ӧ�����ֱ���ʽ������������Ӧ���ͣ�����Ϸ�Ӧ��ֽⷴӦ��B+D��F
̼+����
������̼
| ��ȼ |
̼+����
������̼
��| ��ȼ |
���Ϸ�Ӧ
���Ϸ�Ӧ
��������AΪ��ɫ��Һ������������̺���������ɫҺ��C������B�������ж�AΪ�������⣬CΪˮ��BΪ������C��ͨ������������������������������Կ����ж�HΪ������F�ܹ���������ЧӦ�����Կ����ж�FΪ������̼��DΪ��ɫ���壬���Կ����ж�DΪ̼��GΪ�Ϻ�ɫ���壬�ܹ��������������Կ����ж�GΪ������أ����˸����ʾ��Ѽ�����ϣ�������֤�������⣬���Ծݴ˴��⣮
����⣺��1��AΪ��ɫ��Һ������������̺���������ɫҺ��C������B�������ж�AΪ�������⣬�仯ѧʽΪH2O2��CΪˮ��BΪ������C��ͨ������������������������������Կ����ж�HΪ������F�ܹ���������ЧӦ�����Կ����ж�FΪ������̼���仯ѧʽΪCO2��DΪ��ɫ���壬���Կ����ж�DΪ̼��GΪ�Ϻ�ɫ���壬�ܹ��������������Կ����ж�GΪ������أ��仯ѧʽΪ��KMnO4��
��2�����ݣ�1���еĽ�����֪��BΪ��������������֧��ȼ�գ�����������ȼ����FΪ������̼����̬�Ķ�����̼����Ϊ�ɱ���
��3�����ݣ�1���еĽ�����֪��BΪ������DΪ̼��FΪ������̼���÷�Ӧ�����ֱ���ʽΪ��̼+����
������̼��
�ʴ�Ϊ����1��H2O2��KMnO4��CO2��
��2��������ȼ�����ɱ���
��3��̼+����
������̼��
��2�����ݣ�1���еĽ�����֪��BΪ��������������֧��ȼ�գ�����������ȼ����FΪ������̼����̬�Ķ�����̼����Ϊ�ɱ���
��3�����ݣ�1���еĽ�����֪��BΪ������DΪ̼��FΪ������̼���÷�Ӧ�����ֱ���ʽΪ��̼+����
| ��ȼ |
�ʴ�Ϊ����1��H2O2��KMnO4��CO2��
��2��������ȼ�����ɱ���
��3��̼+����
| ��ȼ |
����������Ϊ��ͼʽ�ƶ��⣬����ؼ��Ǵ���Ϣ����ͻ�ƿڣ�ֱ�ӵó��𰸣�Ȼ����˳�ƻ����ƻ����м��Ƶķ����Ʋ�������𰸣�������ȷ��ɣ�

��ϰ��ϵ�д�
 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д�
�����Ŀ
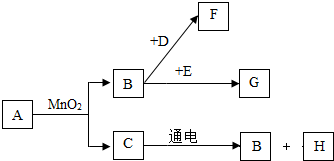
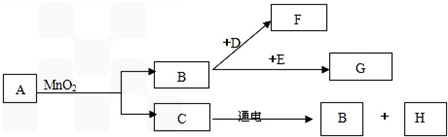
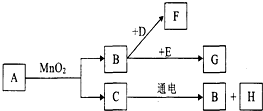
 A��G�dz��г�����7�����ʣ���������ͼ��ʾ��ת����ϵ����֪A��C����ɫҺ�壬B��F��G����ɫ���壬���д����ŷ�F����������ЧӦ��D�Ǻ�ɫ���壬E�ڴ�����B��ȼ�շ�������������ɫ���森
A��G�dz��г�����7�����ʣ���������ͼ��ʾ��ת����ϵ����֪A��C����ɫҺ�壬B��F��G����ɫ���壬���д����ŷ�F����������ЧӦ��D�Ǻ�ɫ���壬E�ڴ�����B��ȼ�շ�������������ɫ���森