��Ŀ����
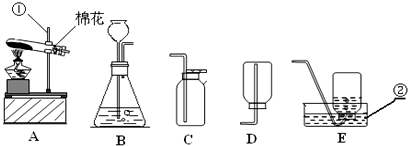 ��������ʵ��װ��ͼ���ش��й����⣺
��������ʵ��װ��ͼ���ش��й����⣺��1��д��������������ƣ�
��
����̨
����̨
��ˮ��
ˮ��
��2�������£��÷ֽ����������Һ��ȡ������ͨ���������������������д����Ӧ�����ֱ���ʽ��
��������
ˮ+����
| �������� |
��������
ˮ+����
����Ӧǰ��������̵ı���������| �������� |
��ѧ����
��ѧ����
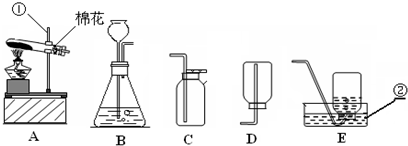
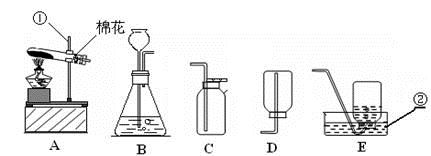
���䣮��3��ʵ�����ø��������ȡ���ռ�����ʱ����ѡ�õ�װ�������
A
A
������ţ����Թܿ����������ǣ���ֹ������ط�ĩ���뵼����
��ֹ������ط�ĩ���뵼����
���Թܿ�ΪʲôҪ������б����ֹ����ˮ�������ȵ��Թ������Թܵ�ը��
��ֹ����ˮ�������ȵ��Թ������Թܵ�ը��
������ˮ���ռ�����ʱ����������������
��������
ʱ����ʼ�ռ�������ˮ���ռ�������Ϻ�ֹͣ����ʱ�IJ���˳�������������ܴ�ˮ�����Ƴ�
�������ܴ�ˮ�����Ƴ�
��Ȼ��Ϩ��ƾ���
Ϩ��ƾ���
���Է�ֹˮ���������ȵ��Թ��У�����Թ����ѣ���4�����ѡ��Aװ����ȡ��������ô���װ�õ������ԣ�
��Ҫ����װ���������Ƚ����ܵ�һ�˷���ˮ�У�������ס�Թܵ���ڣ����װ�ò�©����Ӧ�۲쵽�������� ���ܵ�һ��������ð���������ַſ����ѵ��ܼ���ͣ����ˮ�У���ʱ�ֿɹ۲쵽��������ˮ�������뵼�ܣ��γ�ˮ����
��Ҫ����װ���������Ƚ����ܵ�һ�˷���ˮ�У�������ס�Թܵ���ڣ����װ�ò�©����Ӧ�۲쵽�������� ���ܵ�һ��������ð���������ַſ����ѵ��ܼ���ͣ����ˮ�У���ʱ�ֿɹ۲쵽��������ˮ�������뵼�ܣ��γ�ˮ����
�����������ſ������ռ�����������������������ǵ�ľ�����ڼ���ƿ�ڣ���ľ����ȼ��֤���ռ�����
�������ǵ�ľ�����ڼ���ƿ�ڣ���ľ����ȼ��֤���ռ�����
����������1����Ϥ�������������ƺ���;��
��2������ʵ����Ӧ�÷ֽ����������Һ��ȡ������ԭ�������ش�
��3������ʵ�����ø��������ȡ�ռ�������ԭ�������輰ע�����������ش�
��4�������ܱ�������ѹǿ�ı仯�������������Եķ������������������ʷ��������ķ�����
��2������ʵ����Ӧ�÷ֽ����������Һ��ȡ������ԭ�������ش�
��3������ʵ�����ø��������ȡ�ռ�������ԭ�������輰ע�����������ش�
��4�������ܱ�������ѹǿ�ı仯�������������Եķ������������������ʷ��������ķ�����
����⣺��1����ͼʾ��֪���������������̨������ˮ�ۣ�
��2�������£��÷ֽ����������Һ��ȡ������ͨ�����������������������Ӧ�����ֱ���ʽ����������
ˮ+��������Ӧǰ��������̵ı��������ͻ�ѧ���䣮
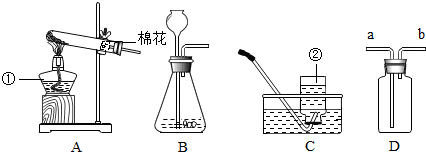
��3��ʵ�����ø��������ȡ���ռ�����ʱ�������Ǽ��ȹ�����ȡ���壬��ѡ�õ�װ�������A��
Ϊ�˷�ֹ������ط�ĩ���뵼���ܣ����Թܿڷ�һ������
Ϊ��ֹ����ˮ�������ȵ��Թ������Թܵ�ը�ѣ��Թܿ�Ҫ������б��
����ˮ���ռ�����ʱ�������ݾ�������ʱ����ʼ�ռ���
����ˮ���ռ�������Ϻ�ֹͣ����ʱ�IJ���˳�����Ƚ������ܴ�ˮ�����Ƴ���Ȼ��Ϩ��ƾ��ƣ��Է�ֹˮ���������ȵ��Թ��У�����Թ����ѣ�
��4�����ѡ��Aװ����ȡ���������װ�õ������Եķ����ǣ���Ҫ����װ���������Ƚ����ܵ�һ�˷���ˮ�У�������ס�Թܵ���ڣ����װ�ò�©����Ӧ�۲쵽�������ǣ����ܵ�һ��������ð���������ַſ����ѵ��ܼ���ͣ����ˮ�У���ʱ�ֿɹ۲쵽��������ˮ�������뵼�ܣ��γ�ˮ�������������ſ������ռ������������ķ����ǣ��������ǵ�ľ�����ڼ���ƿ�ڣ���ľ����ȼ��֤���ռ����ˣ�
�ʴ�Ϊ����1����������̨������ˮ�ۣ�
��2����������
ˮ+��������ѧ���ʣ�
��3��A����ֹ������ط�ĩ���뵼���ܣ���ֹ����ˮ�������ȵ��Թ������Թܵ�ը�ѣ������������������ܴ�ˮ�����Ƴ���Ϩ��ƾ��ƣ�
��4����Ҫ����װ���������Ƚ����ܵ�һ�˷���ˮ�У�������ס�Թܵ���ڣ����װ�ò�©����Ӧ�۲쵽�������ǣ����ܵ�һ��������ð���������ַſ����ѵ��ܼ���ͣ����ˮ�У���ʱ�ֿɹ۲쵽��������ˮ�������뵼�ܣ��γ�ˮ�����������ǵ�ľ�����ڼ���ƿ�ڣ���ľ����ȼ��֤���ռ����ˣ�
��2�������£��÷ֽ����������Һ��ȡ������ͨ�����������������������Ӧ�����ֱ���ʽ����������
| �������� |
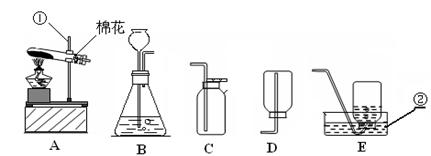
��3��ʵ�����ø��������ȡ���ռ�����ʱ�������Ǽ��ȹ�����ȡ���壬��ѡ�õ�װ�������A��
Ϊ�˷�ֹ������ط�ĩ���뵼���ܣ����Թܿڷ�һ������
Ϊ��ֹ����ˮ�������ȵ��Թ������Թܵ�ը�ѣ��Թܿ�Ҫ������б��
����ˮ���ռ�����ʱ�������ݾ�������ʱ����ʼ�ռ���
����ˮ���ռ�������Ϻ�ֹͣ����ʱ�IJ���˳�����Ƚ������ܴ�ˮ�����Ƴ���Ȼ��Ϩ��ƾ��ƣ��Է�ֹˮ���������ȵ��Թ��У�����Թ����ѣ�
��4�����ѡ��Aװ����ȡ���������װ�õ������Եķ����ǣ���Ҫ����װ���������Ƚ����ܵ�һ�˷���ˮ�У�������ס�Թܵ���ڣ����װ�ò�©����Ӧ�۲쵽�������ǣ����ܵ�һ��������ð���������ַſ����ѵ��ܼ���ͣ����ˮ�У���ʱ�ֿɹ۲쵽��������ˮ�������뵼�ܣ��γ�ˮ�������������ſ������ռ������������ķ����ǣ��������ǵ�ľ�����ڼ���ƿ�ڣ���ľ����ȼ��֤���ռ����ˣ�
�ʴ�Ϊ����1����������̨������ˮ�ۣ�
��2����������
| �������� |
��3��A����ֹ������ط�ĩ���뵼���ܣ���ֹ����ˮ�������ȵ��Թ������Թܵ�ը�ѣ������������������ܴ�ˮ�����Ƴ���Ϩ��ƾ��ƣ�
��4����Ҫ����װ���������Ƚ����ܵ�һ�˷���ˮ�У�������ס�Թܵ���ڣ����װ�ò�©����Ӧ�۲쵽�������ǣ����ܵ�һ��������ð���������ַſ����ѵ��ܼ���ͣ����ˮ�У���ʱ�ֿɹ۲쵽��������ˮ�������뵼�ܣ��γ�ˮ�����������ǵ�ľ�����ڼ���ƿ�ڣ���ľ����ȼ��֤���ռ����ˣ�
����������Ƚ�ȫ�濼����ʵ�����йص��������Ʒ���Ҫ�����������Ŀʱ�����ȣ�Ҫ���������ʵ������ȡԭ�����������ܽ��ԡ��ܶȵȣ���Ҫ��Ϥ������������;��������ʵ�������ע������ȵȣ�

��ϰ��ϵ�д�
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д� ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
�����Ŀ