��Ŀ����
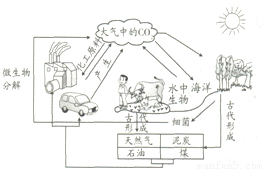
 ͼ��ʾΪ��Ȼ����̼ѭ��ʾ��ͼ��
ͼ��ʾΪ��Ȼ����̼ѭ��ʾ��ͼ����1�������ܹ����ն�����̼����ԭ��֮һ�Ƕ�����̼���Ը�ˮ��Ӧ����д���÷�Ӧ�Ļ�ѧ��ʽ
CO2+H2O=H2CO3
CO2+H2O=H2CO3
����2������֤�˺Ͷ�������������к��ж�����̼���û�ѧ����ʽ��ʾ����
CO2+Ca��OH��2=CaCO3��+H2O
CO2+Ca��OH��2=CaCO3��+H2O
����3�������ͼ����������̼������ͻ�����Ӱ�죨��д��һ�����ɣ���
����
������̼�ٽ�ֲ��������
������̼�ٽ�ֲ��������
���ף�
������̼����������������ЧӦ
������̼����������������ЧӦ
����������1��������̼����ˮ��Ӧ����̼�ᣬ�ݷ�Ӧԭ����д����ʽ��
��2�����ö�����̼��ʹ�����ʯ��ˮ����ǵ����ʽ��
��3����ѧ��һ��˫�н������������������һ�棬Ҳ�в�����һ�棬���ö�����̼��ֲ�������õ�ԭ�ϺͶ�����̼����������ЧӦ���
��2�����ö�����̼��ʹ�����ʯ��ˮ����ǵ����ʽ��
��3����ѧ��һ��˫�н������������������һ�棬Ҳ�в�����һ�棬���ö�����̼��ֲ�������õ�ԭ�ϺͶ�����̼����������ЧӦ���
����⣺��1��������̼��ˮ��Ӧ����̼�ᣬ����ʽ�ǣ�CO2+H2O=H2CO3��
��2��������̼��ʹ�����ʯ��ˮ����ǣ�����̼��ƺ�ˮ����������֤������̼������ʽ��CO2+Ca��OH��2=CaCO3��+H2O��
��3��������̼��ֲ�������õ�ԭ�ϣ�����������һ�棬���ŷ���������������ЧӦ����ƽ����������û���У�
�ʴ�Ϊ����1��CO2+H2O=H2CO3��
��2��CO2+Ca��OH��2=CaCO3��+H2O��
��3������������̼�ٽ�ֲ�������ã�
�ף�������̼����������������ЧӦ��
��2��������̼��ʹ�����ʯ��ˮ����ǣ�����̼��ƺ�ˮ����������֤������̼������ʽ��CO2+Ca��OH��2=CaCO3��+H2O��
��3��������̼��ֲ�������õ�ԭ�ϣ�����������һ�棬���ŷ���������������ЧӦ����ƽ����������û���У�
�ʴ�Ϊ����1��CO2+H2O=H2CO3��
��2��CO2+Ca��OH��2=CaCO3��+H2O��
��3������������̼�ٽ�ֲ�������ã�
�ף�������̼����������������ЧӦ��
������������Ҫ�����˻�ѧ����ʽ����д��������̼������������������ݣ�����������ѧ�Ķ�����̼�����ʽ��з������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
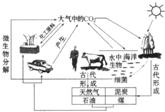 9������Ϊ��Ȼ����̼ѭ��ʾ��ͼ��
9������Ϊ��Ȼ����̼ѭ��ʾ��ͼ��
 ________���ڢ��У����Ķ�����̼�Ĺ��̽���__________���ڢ��У����Ķ�����̼��Ӧ�Ļ�ѧ����ʽΪ______________���ڢ��У�����Ȼ��Ϊ����ȼ�շ�Ӧ�Ļ�ѧ����ʽΪ____________________��
________���ڢ��У����Ķ�����̼�Ĺ��̽���__________���ڢ��У����Ķ�����̼��Ӧ�Ļ�ѧ����ʽΪ______________���ڢ��У�����Ȼ��Ϊ����ȼ�շ�Ӧ�Ļ�ѧ����ʽΪ____________________��