��Ŀ����
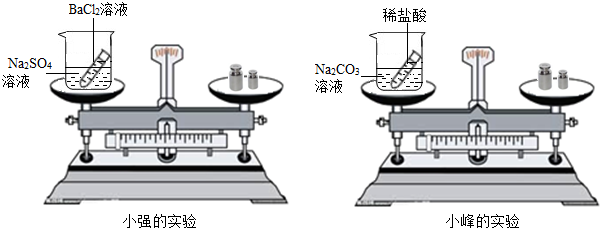
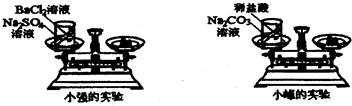
��ѧϰ�������غ㶨�ɺ�С����С������ʵ���ң���������ͼ��ʾ��ʵ��װ�ã���֤������������Һ������ͭ��Һ��Ӧ�Ƿ��������غ㶨�ɵģ���1����д���÷�Ӧ�Ļ�ѧ����ʽ______��
��2��ʵ�����������������ʱС�����֣�ԭ������������Һ�Ƿ��ڳ��������У��������ǶԸո���ɵ�ʵ����������ʣ�
[�������]�����������Ƿ���ʣ�
���������������Һ�Ѿ����ʣ������ʡ�������������Һ������ͭ��Һ��ӦΪʲô��Ȼ���������غ㶨�ɣ�
[��������]����ͭ�ε��ܽ��Ա���20�棩
| ������������ | SO42- | NO3- | PO43- | Cl- | CO32- |
| Cu2+ | �� | �� | �� | �� | �� |
����������ѧ֪ʶ��������������Ʒ��Һ�����ʳɷ��������²��룺
[����]�ٿ�����NaOH�� �ڿ�����Na2CO3�� �ۿ�����NaOH��Na2CO3�Ļ���
[���ʵ��]��ȡ������Ʒ��Һ���μ�ϡ���ᣬ���������ɣ��ɴ���Ϊ�������ȷ�����жϴ˽����Ƿ���ȷ�����������ɣ�______��
��ȡ������Ʒ��Һ���μӷ�̪��Һ����Һ��Ϊ��ɫ���ɴ���Ϊ�������ȷ�����жϴ˽����Ƿ���ȷ�����������ɣ�______��
��ȡ������Ʒ��Һ���μ�BaCl2��Һ���а�ɫ�����������ɴ��жϰ�ɫ��ĩ�к���______��Ϊ����֤����ۣ���������Һ�еμ�BaCl2��Һ�����ٲ���������Ȼ����ˣ���������Ӧ���е�ʵ����______�����۲쵽______������������ȷ��
[���������]�ټ������������ѱ��ʣ�
�ڡ����ʡ�������������Һ������ͭ��Һ��Ӧ��Ϊʲô��Ȼ���������غ㶨�ɣ�______��
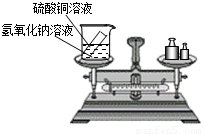
���𰸡���������1���÷�Ӧ�Ǽ���η����ĸ��ֽⷴӦ��
��3���ɸ����������Ƶı�����Ϣ��������ʺ��������̼���ƣ����Կ�ѡ���Լ�������̼���Ʒ�Ӧ�������������ʣ�
�ڡ����ʡ�������������Һ������ͭ��Һ��Ӧ��Ȼ���������غ㶨�ɣ�
����⣺��1���÷�Ӧ�Ǽ���η����ĸ��ֽⷴӦ����ѧ����ʽΪ��2NaOH+CuSO4=Na2SO4+Cu��OH��2����
��3��[���ʵ��]����ȷ���������Ƶı��������������������̼�ķ�Ӧ�������к���̼���ƣ��������������ɶ�����̼�����ܶ϶���̼����һ�����ʻ��ǻ���̼���Ƶ��������ƣ�
����ȷ��̼������ҺҲ�Լ��ԣ���ʹ��ɫ�ķ�̪���ɫ��
��ȡ������Ʒ��Һ���μ�BaCl2��Һ���а�ɫ�����������ɴ��жϰ�ɫ��ĩ�к���̼���ƣ�Ϊ����֤����ۣ���������Һ�еμ�BaCl2��Һ�����ٲ���������Ȼ����ˣ���������Ӧ���е�ʵ���ǵμ���ɫ�ķ�̪��Һ�����۲쵽��Һ���ɫ������������ȷ��
��̼������Һ������ͭ��Һ����Ӧ������û�������ݳ�����̼������Һ������ͭ��Һ��Ӧ����Ӧ������������������ձ��У���
�ʴ�Ϊ��
��1��2NaOH+CuSO4=Na2SO4+Cu��OH��2����
��3������ȷ �������Ҳ�ɢ���ȷ ̼������ҺҲ�ʼ��Ԣ�Na2CO3����Һ�еμӷ�̪��Һ ��Һ��죻
��̼������Һ������ͭ��Һ����Ӧ������û�������ݳ�����̼������Һ������ͭ��Һ��Ӧ����Ӧ������������������ձ��У���
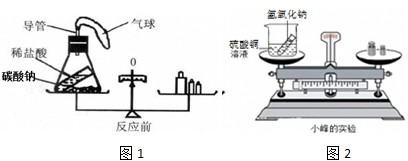
������������������ڶ������غ㶨�ɽ���ʵ��̽��ʱ��Ҫѡ���ܱյ�װ�û�����ѡ��ҩƷʱҪע�ⲻҪѡ��Ӧǰ���������������ɵķ�Ӧ��
��3���ɸ����������Ƶı�����Ϣ��������ʺ��������̼���ƣ����Կ�ѡ���Լ�������̼���Ʒ�Ӧ�������������ʣ�
�ڡ����ʡ�������������Һ������ͭ��Һ��Ӧ��Ȼ���������غ㶨�ɣ�
����⣺��1���÷�Ӧ�Ǽ���η����ĸ��ֽⷴӦ����ѧ����ʽΪ��2NaOH+CuSO4=Na2SO4+Cu��OH��2����
��3��[���ʵ��]����ȷ���������Ƶı��������������������̼�ķ�Ӧ�������к���̼���ƣ��������������ɶ�����̼�����ܶ϶���̼����һ�����ʻ��ǻ���̼���Ƶ��������ƣ�
����ȷ��̼������ҺҲ�Լ��ԣ���ʹ��ɫ�ķ�̪���ɫ��
��ȡ������Ʒ��Һ���μ�BaCl2��Һ���а�ɫ�����������ɴ��жϰ�ɫ��ĩ�к���̼���ƣ�Ϊ����֤����ۣ���������Һ�еμ�BaCl2��Һ�����ٲ���������Ȼ����ˣ���������Ӧ���е�ʵ���ǵμ���ɫ�ķ�̪��Һ�����۲쵽��Һ���ɫ������������ȷ��
��̼������Һ������ͭ��Һ����Ӧ������û�������ݳ�����̼������Һ������ͭ��Һ��Ӧ����Ӧ������������������ձ��У���
�ʴ�Ϊ��
��1��2NaOH+CuSO4=Na2SO4+Cu��OH��2����
��3������ȷ �������Ҳ�ɢ���ȷ ̼������ҺҲ�ʼ��Ԣ�Na2CO3����Һ�еμӷ�̪��Һ ��Һ��죻
��̼������Һ������ͭ��Һ����Ӧ������û�������ݳ�����̼������Һ������ͭ��Һ��Ӧ����Ӧ������������������ձ��У���
������������������ڶ������غ㶨�ɽ���ʵ��̽��ʱ��Ҫѡ���ܱյ�װ�û�����ѡ��ҩƷʱҪע�ⲻҪѡ��Ӧǰ���������������ɵķ�Ӧ��

��ϰ��ϵ�д�
�����Ŀ
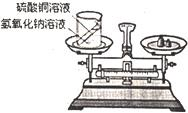 24����ѧϰ�������غ㶨�ɺ�С����С������ʵ���ң���������ͼ��ʾ��ʵ��װ�ã���֤������������Һ������ͭ��Һ��Ӧ�Ƿ��������غ㶨�ɵģ�
24����ѧϰ�������غ㶨�ɺ�С����С������ʵ���ң���������ͼ��ʾ��ʵ��װ�ã���֤������������Һ������ͭ��Һ��Ӧ�Ƿ��������غ㶨�ɵģ�