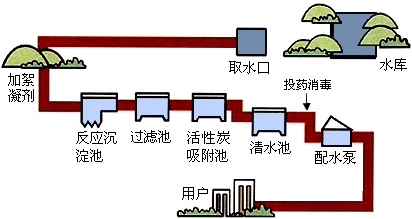
��Ŀ����
23��2010�ꡰ����ˮ�ա��������ǡ���עˮ�ʡ�ץס������Ӧ����ս�����˽�ˮ������ˮ����Լˮ��ÿ������Ӧ�������Σ�
��1��ˮ����
��Ϊ�˷�ֹú���ж������������ڷ�һ��ˮ
��ͼ�����ͼ���Ż������ˮ����
�۳�������Ӳˮ�Dz��Ƶ���÷��������������彡��
���Ϻ�������ˮ����ʹ֮Ư���ڿ����У�����������
��2����Լ��ˮ����ˮ������Ϊ��Ҫ�������DZ�������ˮ�ʵĽ��飬���в���������
���Ͻ�������ˮ�������
�ڼ���ž�����ũ��ʹ��ũҩ�ͻ���
���Ͻ��ںӱ�����ѷ���������
���Ͻ��ں��С��Ӱ�����������Ǧ�Ƚ����ķϾɵ��
��3��ʵ����������ʳ��ˮ������ˮ������
��4�����ˮʱ��ˮ�м���ϡ���������������Һ��Ŀ����
��5�������н�Լ��ˮ�ķ����ܶ࣬�磺��ϴ��ˮ������������پ�һ����
��1��ˮ����
������
��������������ʡ������������ˮ���ճ����������Ź㷺��Ӧ�ã�����Ӧ����ȷ������
�������ִ��ţ�����Ϊ�˷�ֹú���ж������������ڷ�һ��ˮ
��ͼ�����ͼ���Ż������ˮ����
�۳�������Ӳˮ�Dz��Ƶ���÷��������������彡��
���Ϻ�������ˮ����ʹ֮Ư���ڿ����У�����������
��2����Լ��ˮ����ˮ������Ϊ��Ҫ�������DZ�������ˮ�ʵĽ��飬���в���������
��
�������ִ��ţ����Ͻ�������ˮ�������
�ڼ���ž�����ũ��ʹ��ũҩ�ͻ���
���Ͻ��ںӱ�����ѷ���������
���Ͻ��ں��С��Ӱ�����������Ǧ�Ƚ����ķϾɵ��
��3��ʵ����������ʳ��ˮ������ˮ������
����
������������ˡ����ķ�������4�����ˮʱ��ˮ�м���ϡ���������������Һ��Ŀ����
��ǿ������
����5�������н�Լ��ˮ�ķ����ܶ࣬�磺��ϴ��ˮ������������پ�һ����
������ˮ��������ϴ��ˮϴ�ϰѡ����ֹغ�����ˮ��ͷ�ȣ�
����������1������ˮ����ɣ��������ʷ�������жϣ����ݶ�ˮ����ʶ���жϹ���ˮ��Ӧ�������ں���Ӧ�õ�ѡ�
��2������ˮ��Ⱦ����ε�֪ʶ���ж�������Ľ����У����Ͻ�Լ��ˮ����ˮ�����ĺ��������飻
��3������ʳ��ˮ������ˮ����ϵIJ�ͬ����ƿ�����������ˮ��ʵ�鷽����
��4�����ݶԵ��ˮʵ������⣬˵���ڵ��ˮʱ����ϡ���������������Һ��Ŀ�ģ�
��5���������ʵ�ʣ��ٳ���Լ��ˮ��������
��2������ˮ��Ⱦ����ε�֪ʶ���ж�������Ľ����У����Ͻ�Լ��ˮ����ˮ�����ĺ��������飻
��3������ʳ��ˮ������ˮ����ϵIJ�ͬ����ƿ�����������ˮ��ʵ�鷽����
��4�����ݶԵ��ˮʵ������⣬˵���ڵ��ˮʱ����ϡ���������������Һ��Ŀ�ģ�
��5���������ʵ�ʣ��ٳ���Լ��ˮ��������
����⣺
��1��ˮ����H��O����Ԫ����ɵ��������ú����ҪΪһ����̼�������岻����ˮ�������ڷ�һ��ˮ�����ܷ�ֹú���ж���˵������ȷ����ͼ�����ͼ���Ż���ˮ��������ͼ�����٣�������ѷ������۳�������Ӳˮ�����������彡����˵������ȷ���ܽ�ˮ����ʹ֮Ư���ڿ����У�ʹˮ�����������������������ã�˵����ȷ��
��2������ž�����ũ��ʹ��ũҩ�ͻ��ʣ����к�����ʵ�ʣ�ֻ���Ǻ���ʹ�ã����ܶž�ʹ�ã��ʸý��鲻������
��3��ͨ��������ʳ��ˮ�����¹��������ˮ�����κκۼ�����˿������ֳ�ʳ��ˮ������ˮ��
��4��ˮ�ĵ��������ܲΪ��ǿˮ�ĵ����ԣ����ڵ��ˮʱ��ˮ�м�������������������ƣ�
��5��һˮ���ã���������ˮ��������ϴ��ˮϴ�ϰѵȣ�������Ч�ؽ�Լ��ˮ��
�ʴ�Ϊ��
��1��������ܣ���2���ڣ���3����������4����ǿ�����ԣ���5��������ˮ��������ϴ��ˮϴ�ϰѡ����ֹغ�����ˮ��ͷ�ȣ���
��1��ˮ����H��O����Ԫ����ɵ��������ú����ҪΪһ����̼�������岻����ˮ�������ڷ�һ��ˮ�����ܷ�ֹú���ж���˵������ȷ����ͼ�����ͼ���Ż���ˮ��������ͼ�����٣�������ѷ������۳�������Ӳˮ�����������彡����˵������ȷ���ܽ�ˮ����ʹ֮Ư���ڿ����У�ʹˮ�����������������������ã�˵����ȷ��
��2������ž�����ũ��ʹ��ũҩ�ͻ��ʣ����к�����ʵ�ʣ�ֻ���Ǻ���ʹ�ã����ܶž�ʹ�ã��ʸý��鲻������
��3��ͨ��������ʳ��ˮ�����¹��������ˮ�����κκۼ�����˿������ֳ�ʳ��ˮ������ˮ��
��4��ˮ�ĵ��������ܲΪ��ǿˮ�ĵ����ԣ����ڵ��ˮʱ��ˮ�м�������������������ƣ�
��5��һˮ���ã���������ˮ��������ϴ��ˮϴ�ϰѵȣ�������Ч�ؽ�Լ��ˮ��
�ʴ�Ϊ��
��1��������ܣ���2���ڣ���3����������4����ǿ�����ԣ���5��������ˮ��������ϴ��ˮϴ�ϰѡ����ֹغ�����ˮ��ͷ�ȣ���
����������Ϊ����ˮ�й�֪ʶ�Ŀ��飬��Ŀ�ѶȲ����漰֪ʶ��϶࣬�������ڹ��ɡ��Աȵ�ϰ�߶��ڽ������������нϴ������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ



 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��2010?��ƽ��2010�ꡰ����ˮ�ա��������ǡ������ˮ���������硱��
��2010?��ƽ��2010�ꡰ����ˮ�ա��������ǡ������ˮ���������硱��