��Ŀ����
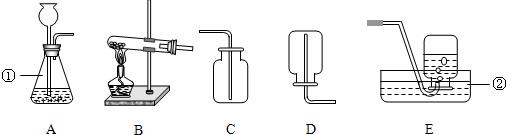
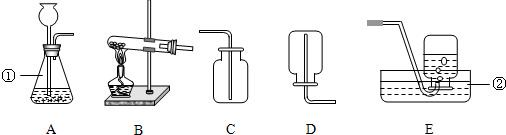
��ѧС���ͬѧ������ȡһ�����壮ʵ��������ϡ���ᡢϡ���ᡢ��״ʯ��ʯ���Լ�����������
��1�����������ƣ�______�����������ƣ�______��
��2������ҩƷѡ����ȡ����������ķ���װ��Ϊ��______������ĸ�����ռ�װ��Ϊ��______��������ĸ��
��3��д��ʵ������ȡ�����巴Ӧ�Ļ�ѧ����ʽ��______��
��4�����������ɲ��ó����ʯ��ˮ�����ǻ�ѧС���ͬѧ��ȡ��ʱ�����Լ�ƿ������һ���Ĥ������Ĥ���γ�ԭ���ǣ�
______���û�ѧ����ʽ��ʾ����
��5���е�ͬѧ�������ͼװ����Ϊ��ȡ������ķ���װ�ã���װ���к��ŵ㣿______����дһ�㼴�ɣ�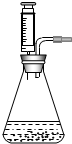

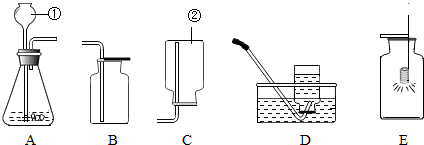
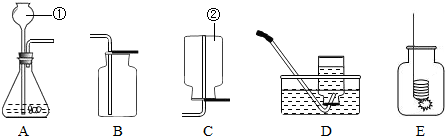
��1�����������ƣ�______�����������ƣ�______��
��2������ҩƷѡ����ȡ����������ķ���װ��Ϊ��______������ĸ�����ռ�װ��Ϊ��______��������ĸ��
��3��д��ʵ������ȡ�����巴Ӧ�Ļ�ѧ����ʽ��______��
��4�����������ɲ��ó����ʯ��ˮ�����ǻ�ѧС���ͬѧ��ȡ��ʱ�����Լ�ƿ������һ���Ĥ������Ĥ���γ�ԭ���ǣ�
______���û�ѧ����ʽ��ʾ����
��5���е�ͬѧ�������ͼװ����Ϊ��ȡ������ķ���װ�ã���װ���к��ŵ㣿______����дһ�㼴�ɣ�

�𣺣�1��ʶ���������ʴ�Ϊ����ƿ�� ˮ�ۣ�
��2������ҩƷֻ���ƶ�����̼���壬ҩƷ��ϡ����Ϳ�״ʯ��ʯ������װ�����ڹ����Һ�岻���������壮������̼�ܶȱȿ������������ſ������ռ����ʴ�Ϊ��A�� C��
��3������ʵ�����ƶ�����̼��֪���ʴ�Ϊ��CaCO3+2HCl=CaCl2+H2O+CO2����
��4��������̼��ʯ��ˮ����̼��ƣ�̼��Ʋ�����ˮ���ʴ�Ϊ��Ca��OH��2+CO2=CaCO3��+H2O��
��5���ȽϿ��Է��֣���ͼ��һ��ע���������Ը�����Ҫ���ڼ���Һ��Ŀ�������ȻҲ������ʱֹͣ���ʴ�Ϊ�����Կ��Ʒ�Ӧ�Ŀ�������������Ҳ�У���
��2������ҩƷֻ���ƶ�����̼���壬ҩƷ��ϡ����Ϳ�״ʯ��ʯ������װ�����ڹ����Һ�岻���������壮������̼�ܶȱȿ������������ſ������ռ����ʴ�Ϊ��A�� C��
��3������ʵ�����ƶ�����̼��֪���ʴ�Ϊ��CaCO3+2HCl=CaCl2+H2O+CO2����
��4��������̼��ʯ��ˮ����̼��ƣ�̼��Ʋ�����ˮ���ʴ�Ϊ��Ca��OH��2+CO2=CaCO3��+H2O��
��5���ȽϿ��Է��֣���ͼ��һ��ע���������Ը�����Ҫ���ڼ���Һ��Ŀ�������ȻҲ������ʱֹͣ���ʴ�Ϊ�����Կ��Ʒ�Ӧ�Ŀ�������������Ҳ�У���

��ϰ��ϵ�д�
�����Ŀ
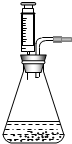 29����ѧС���ͬѧ������ȡһ�����壮ʵ��������ϡ���ᡢϡ���ᡢ��״ʯ��ʯ���Լ�����������
29����ѧС���ͬѧ������ȡһ�����壮ʵ��������ϡ���ᡢϡ���ᡢ��״ʯ��ʯ���Լ�����������