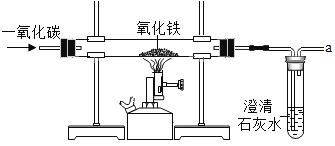
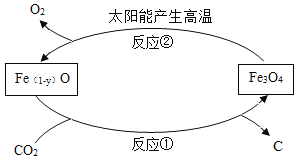
��Ŀ����
����Ŀ����ͼ��ʵ���ҳ���������������Щ��������ɶ��ʵ�飬���ͼ�ش����⡣
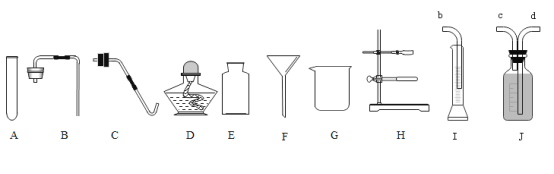
��1������Һ�壺��Ҫ�õ�������D��������______��
��2������Һ�壺���������⣬����ѡ������G��H��____������ĸ����
��3����ȡ���壺ʵ������ȡ������̼�Ļ�ѧ����ʽΪ__________����װ��ȡ���ռ�������̼��װ�ã��������������ѡ��____________������ĸ����ʵ�����ø��������ȡ�����Ļ�ѧ����ʽΪ__________����������������װ����װ�ã���Ҫ�õ���������_________������ĸ����
��4��С���ó���ʯ��ˮ���������̼����ԭ����_________���û�ѧ����ʽ��ʾ����
��5��С��������ͼ����I��J��ʾװ���ռ�һ��������������װ�ýӿڵ�����˳��Ϊ____________����Ͳ��������_________________��
���𰸡��ƾ��� F CaCO3��2HCl = CaCl2��H2O��CO2�� ABEH 2KMnO4 ![]() K2MnO4��MnO2��O2�� ACDH CO2��Ca��OH��2 = CaCO3����H2O cdb ��Ӳ������������
K2MnO4��MnO2��O2�� ACDH CO2��Ca��OH��2 = CaCO3����H2O cdb ��Ӳ������������
��������
��1����ͼ��֪����D�Ǿƾ��ƣ�
��2�����˲�����Ҫ��������©�����ձ�������̨������F��
��3����ʵ�����У����ô���ʯ��ʯ��ʯ��ϡ���ᷴӦ����ȡ������̼����̼��������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2������ȡ������̼�ķ���װ�����ڹ�Һ�����ͣ�������̼������ˮ���ܶȱȿ����������������ſ������ռ�������ѡ��ABEH��װ����ȡ���ռ�������̼��װ�ã���������ڼ��ȵ������·ֽ�Ϊ����ء��������̺���������Ӧ�Ļ�ѧ����ʽΪ��2KMnO4 ![]() K2MnO4��MnO2��O2�����÷�Ӧ���ڹ�������ͣ�����ѡ������ACDH����װ��
K2MnO4��MnO2��O2�����÷�Ӧ���ڹ�������ͣ�����ѡ������ACDH����װ��
��4��������̼���������Ʒ�Ӧ����̼��Ƴ�����ˮ����Ӧ�Ļ�ѧ����ʽΪ��CO2+Ca��OH��2 = CaCO3��+H2O��
��5��С��Ϊ����ͼI��Jװ���ռ�һ������������װ�ýӿ����ӵ�˳��Ϊ��cbd����Ͳ���ռ���ˮ�������Ϊ�ռ�����������������Ͳ���������ж��ռ������������