��Ŀ����
ˮ������֮Դ��Ҳ������������Դ��������ѧ���Ļ�ѧ֪ʶ���ش��������⣺
(1)��Լˮ��Դ����ֹˮ��Ⱦ��ÿ������Ӧ�������κ������������������ˮ����Ⱦ���У�����ţ� ��
A����ҵ��ˮֱ���ŷ� B����ҵ�����������ŷ�
C����ֹʹ�ú���ϴ�·� D������ʹ�û��ʡ�ũҩ
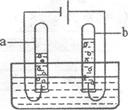
(2)����ͼ��ˮͨ��ֽ��ʾ��ͼ����ʵ������У��Թ�a�в����������� ��д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
(3)Ϊ��ȥˮ�еIJ��������ʣ�ijͬѧ��������ͼ��ʾ�ļ���ˮ�������л���̿����Ҫ������ ��
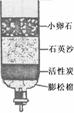 (4)ijЩ�ط�������ˮ�к���������Ca(HCO3)2�ȿ������Ρ���ˮʱ��Ca(HCO3)2�����ֽⷴӦ�����������Ե�CaCO3����д��Ca(HCO3)2���ȷֽ�Ļ�ѧ����ʽ ��
(4)ijЩ�ط�������ˮ�к���������Ca(HCO3)2�ȿ������Ρ���ˮʱ��Ca(HCO3)2�����ֽⷴӦ�����������Ե�CaCO3����д��Ca(HCO3)2���ȷֽ�Ļ�ѧ����ʽ ��
(1)A��D (2)����(��H2) 2H2O ![]() 2H2��+ O2��
2H2��+ O2��
(3)���� (4) Ca(HCO3)2 ��== CaCO3��+ CO2��+ H2O

��ϰ��ϵ�д�
�����Ŀ
 ˮ������֮Դ��Ҳ������������Դ��������ѧ���Ļ�ѧ֪ʶ���ش��������⣺
ˮ������֮Դ��Ҳ������������Դ��������ѧ���Ļ�ѧ֪ʶ���ش��������⣺ ˮ������֮Դ��Ҳ������������Դ��������ѧ���Ļ�ѧ֪ʶ���ش��������⣺
ˮ������֮Դ��Ҳ������������Դ��������ѧ���Ļ�ѧ֪ʶ���ش��������⣺