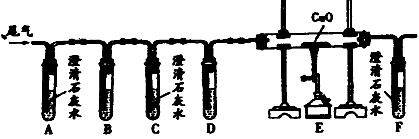��Ŀ����
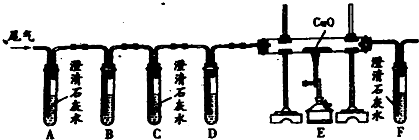
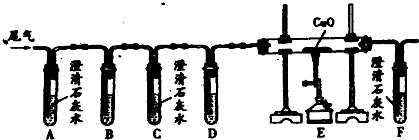
��ѧ��ȤС���ͬѧ�������һ����̼���廹ԭ��������ʵ��װ�ã���ͼ��ʾ���ش��������⣺
��1��Ϊ�˱��ⲣ����a�ڼ���ʱ���ܷ�����ը������ǰӦ������
��2��a����Fe2O3������Ӧ�Ļ�ѧ����ʽΪ������
��3��Bװ�ó�������CO2�����⣬��������д��Bװ����NaOH������Ӧ�Ļ�ѧ����ʽ����
��4����a����������ȫ����ԭ��������ȴ��������������ȷ�Ӧǰ������������������2.4g�������a���е�������������������g��

| ��1������һ����̼���п�ȼ�ԣ��������ϣ����ȿ��ܻᷢ����ը���з����� ��2��һ����̼�ڸ���������������Ӧ�������Ͷ�����̼���ݴ���д����ʽ�� ��3�������������Ƶ����ʣ�װ��C�е�����������Һ����β���е� ��4��һ����̼��ԭ��������Ӧ������������ٵ�������Ϊ����������Ԫ�ص�������������������������Ԫ�ص�������ϵ������Ԫ�ص������������������������������������������������ͨ������������Ԫ�ص������������㣮 | |
| ��� | �⣺��1��һ����̼���п�ȼ�ԣ��������ϣ����ȿ��ܻᷢ����ը�������ڼ���ǰҪ��ͨһ����̼�ž��������ڿ�����ֹһ����̼����������ը�� ��2���ڸ��������£�һ����̼����������Ӧ�������Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ3CO+Fe2O3 ��3��װ��C��ʢ������������Һ���Գ�ȥβ���еĶ�����̼��ͬʱһ����̼�����ų�װ���ڵ���Һ�����ռ�����ֹ��ɿ�����Ⱦ��������̼������������Һ��Ӧ����̼���ƺ�ˮ������ʽ�ǣ�CO2+2NaOH═Na2CO3+H2O�� ��4��������Fe2O3��Fe��OԪ�ص�������=��56��2������16��3��=7��3����ˣ�����������Ԫ������Ϊ2.4gʱ����Ԫ�ص�����=2.4g��=5.6g����������������Ϊ��2.4g+5.6g=8g�� �ʴ�Ϊ����1����ͨһ����̼�ž��������ڿ����� ��2��3CO+Fe2O3 ��3�����ռ�������һ����̼����ֹһ����̼�ݳ���Ⱦ������CO2+2NaOH═Na2CO3+H2O�� ��4��8�� |

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д� ������̼ȫ����ȥ��ͬʱ��ʣ���һ����̼�����ռ����Է�ֹһ����̼�ų�����Ⱦ���������ݷ�Ӧԭ����д����ʽ��
������̼ȫ����ȥ��ͬʱ��ʣ���һ����̼�����ռ����Է�ֹһ����̼�ų�����Ⱦ���������ݷ�Ӧԭ����д����ʽ�� 2Fe+3CO2��
2Fe+3CO2��