��Ŀ����
����Ŀ������������գ�
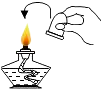
��1��ȡ������������ۻ�ϣ��ѻ����Ž��Թ��ڣ��þƾ��Ƽ��ȣ�����Ӧ����չ����֧�Թ�ʱ������ֹͣ���ȣ�������ȴ��۲쵽�����˺�ɫ���壮 
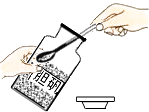
��2��ʵ����ͨ����������Ӧ����������������FeS�������ϡ�����ڳ����·������ֽⷴӦ��ȡ���⣨H2S�����壮������������������ɫ���г�������ζ���ж����壬�ܶȱȿ�����������ˮ��ˮ��Һ��һ���ᣮ��ѡȡ���ʵ���ȡ���ռ�װ��Ϊ������ţ���д����������������Һ������������Ļ�ѧ����ʽ ��
���𰸡�
��1��������
��2��BE��H2S+2NaOH=Na2S+2H2O
���������⣺��1��ȡ������������ۻ�ϣ��ѻ����Ž��Թ��ڣ��þƾ��Ƽ��ȣ�����Ӧ����������չ����֧�Թ�ʱ������ֹͣ���ȣ�������ȴ��۲쵽�����˺�ɫ���壻���Դ��ǣ��������2��ʵ����ͨ������������FeS�������ϡ�����ڳ����·������ֽⷴӦ��ȡ���⣨H2S�����壬��˲���Ҫ���ȣ�������������������ɫ���г�������ζ���ж����壬�ܶȱȿ�����������ˮ�����Ҫ��װ�õij��ܽ��룻���Դ��ǣ�BE��H2S+2NaOH=Na2S+2H2O��
�����㾫����������Ŀ����֪���������ý������ϵ�ѡ�����ݺ���д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ�����֪ʶ���Եõ�����Ĵ𰸣���Ҫ���պ�ɫ������ͨ��ָ�����̡��������ǵĺϽ��ؽ�������ͭ��п��Ǧ�ȣ���ɫ��������������ơ�þ�����ȣ���ɫ������ͨ����ָ����ɫ�������������������ע�⣺a����ƽ b������ c�����ţ�

 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�