��Ŀ����
��7�֣��а�����2011��2��15�ձ��������ճ���������ʯӢ��ԴΪ��������������ǧ��Ԫ���ҵ��ʯӢ����Ҫ�ɷ�Ϊ�������裬���Dz�����ҵ���մɹ�ҵ��ԭ�ϣ�ұ��ҵ�����ۼ���
��1����������Ľṹ����ʯ���ƣ������� ������ӡ�����ԭ�ӡ��������ӡ������ɵģ������������� ����ᡱ����������Ρ������������
��2��װ������������Һ���Լ�ƿ�����ò�������ԭ�����ڳ����£�NaOH�벣�����е�SiO2�����ط�����Ӧ����Na2SiO3��H2O��Na2SiO3ʹƿ����ƿ��ճ����һ����÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3������̫���ܵ�غ͵���оƬ����ȱ�ٵIJ��ϡ������ߴ��������ʾ��ͼ���£�
�� �Ʊ��ֹ�ķ�ӦΪ��SiO2 + 2C Si + 2CO������Ӧ�Ļ���������
Si + 2CO������Ӧ�Ļ���������
�� �����Ʊ����̱���ﵽ��ˮ����������H2��ԭSiHCl3�����л���O2����������ĺ���� ��
�� Ϊ�˴ﵽ��ɫ��ѧ�ͽ�Լ��Դ��Ŀ�ģ�����A��Ҫѭ��ʹ�ã�A�Ļ�ѧʽ��
��7�֣���1��ԭ�� ������ ��2��2NaOH + SiO2 = Na2SiO3 + H2O
��3���û���Ӧ �豻�����ò����ߴ��裬�ҷ�����ը HCl
����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�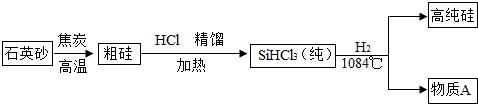

 Si + 2CO������Ӧ�Ļ���������
Si + 2CO������Ӧ�Ļ���������

 Si + 2CO������Ӧ�Ļ���������
Si + 2CO������Ӧ�Ļ���������
