��Ŀ����
��2009?��������ģ������ͼʾʵ��ش��������⣮

��1������a������
��ͨ�������̼ ��ͨ������ �ۼ�������
�ܼ��백ˮ �ݼ����Ȼ��� ��ˮ
��2��Bװ���з�����Ӧ�Ļ�ѧ����ʽΪ
��3��Cװ���У�ȼ�ճ��е�ȼ�ĺ��ף�������Ӧ�Ļ�ѧ����ʽΪ
����ֹˮ�У�����ƿ�н���Һ��������Լռ����ƿ�����1/5����˵��������
��4����Cװ�õļ���ƿ���Ƕ�����̼�������ȼ��þ�����۲쵽��������þ������ȼ�գ���Ӧ��ȫ����ƿ�ײ��а�ɫ�ͺ�ɫ���壬�Ʋ�þ�������̼��Ӧ�Ļ�ѧ����ʽ

��1������a������
�Թ�
�Թ�
��Aװ���У������ͨ���������ʣ���ɫʯ����ɫ���ǣ�����ţ��٢�
�٢�
����ͨ�������̼ ��ͨ������ �ۼ�������
�ܼ��백ˮ �ݼ����Ȼ��� ��ˮ
��2��Bװ���з�����Ӧ�Ļ�ѧ����ʽΪ
2H2O
2H2��+O2��
| ||
2H2O
2H2��+O2��
���������������Թ��в�������ļ��鷽��Ϊ
| ||
�������ǵ�ľ�������ܿڣ��۲쵽ľ����ȼ
�������ǵ�ľ�������ܿڣ��۲쵽ľ����ȼ
����3��Cװ���У�ȼ�ճ��е�ȼ�ĺ��ף�������Ӧ�Ļ�ѧ����ʽΪ
4P+5O2
2P2O5
| ||
4P+5O2
2P2O5
��
| ||
����ֹˮ�У�����ƿ�н���Һ��������Լռ����ƿ�����1/5����˵��������
���������ռ
| 1 |
| 5 |
���������ռ
��| 1 |
| 5 |
��4����Cװ�õļ���ƿ���Ƕ�����̼�������ȼ��þ�����۲쵽��������þ������ȼ�գ���Ӧ��ȫ����ƿ�ײ��а�ɫ�ͺ�ɫ���壬�Ʋ�þ�������̼��Ӧ�Ļ�ѧ����ʽ
2Mg+CO2
2MgO+C
| ||
2Mg+CO2
2MgO+C
��
| ||
�������������е�֪ʶ���з�������ɫʯ����Һ��������Һ��죬���ˮ��������������������������ʹ�ô����ǵ�ľ��������ȼ�����������������ף��ڿ���������Լռ��������������֮һ��������̼����þ��Ӧ��������þ��̼��
����⣺��1����ͼ��֪��a���Թܣ�ʯ����������Һ��죬������̼��ˮ��Һ�����ԣ���������ԣ�����Թܣ��٢ۣ�
��2��Bװ��Ϊ���ˮ��װ�ã�ˮ�ֽܷ�������������������������ʹ�ô����ǵ�ľ�������2H2O
2H2��+O2�����������ǵ�ľ�������ܿڣ��۲쵽ľ����ȼ��
��3������ȼ�����������������ף��ڿ���������Լռ��������������֮һ�����4P+5O2
2P2O5�����������ռ
��
��4����ѧ��Ӧǰ��Ԫ�ص�����䣬þ�������̼��Ӧ���ɵİ�ɫ����������þ����ɫ������̼�����2Mg+CO2
2MgO+C��
��2��Bװ��Ϊ���ˮ��װ�ã�ˮ�ֽܷ�������������������������ʹ�ô����ǵ�ľ�������2H2O
| ||
��3������ȼ�����������������ף��ڿ���������Լռ��������������֮һ�����4P+5O2
| ||
| 1 |
| 5 |
��4����ѧ��Ӧǰ��Ԫ�ص�����䣬þ�������̼��Ӧ���ɵİ�ɫ����������þ����ɫ������̼�����2Mg+CO2
| ||
���������⿼���˳������ʵ����ʣ���ɴ��⣬�����������е�֪ʶ���У���д��Ӧ�Ļ�ѧ����ʽҪע����ƽ��

��ϰ��ϵ�д�
�����Ŀ
 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ̼ԭ�ӣ�
��ʾ̼ԭ�ӣ� ��ʾ��ԭ�ӣ����ƶ�����˵������ȷ���ǣ�������
��ʾ��ԭ�ӣ����ƶ�����˵������ȷ���ǣ�������
 ����X��ֵΪ
����X��ֵΪ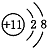 B��
B�� C��
C�� D��
D��