��Ŀ����
�����������ĸ�ף���嫵ĺ�������������ˮ��������̲��ŷḻ�Ļ�ѧ��Դ�����Ȼ��ơ��Ȼ��ơ��Ȼ�þ���廯þ�ȴ��������Σ�
��1����������ּ���ˮ������ˮ�ķ��������������ͻ�ѧ������ֻ��˵��ԭ������
����������______��
��ѧ������______��
��2����ˮ��ɹ�Ρ��õ��Ĵ����к�����������ɳ�Ȳ��������ʣ��ɲ�ȡ���в����ȥ������������������Ӧ�IJ������裮�� �ܽ� ������ ���� ����______��
��3���Ժ�ˮ��ʯ����Ϊԭ����ȡ����þ����������Ϊ������ֱ�д������������þ��þ�Ļ�ѧ��Ӧ����ʽ��
��1������������þ��______��
��2���� �� þ��______��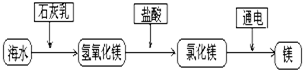
��1����������ּ���ˮ������ˮ�ķ��������������ͻ�ѧ������ֻ��˵��ԭ������
����������______��
��ѧ������______��
��2����ˮ��ɹ�Ρ��õ��Ĵ����к�����������ɳ�Ȳ��������ʣ��ɲ�ȡ���в����ȥ������������������Ӧ�IJ������裮�� �ܽ� ������ ���� ����______��
��3���Ժ�ˮ��ʯ����Ϊԭ����ȡ����þ����������Ϊ������ֱ�д������������þ��þ�Ļ�ѧ��Ӧ����ʽ��
��1������������þ��______��
��2���� �� þ��______��

��1������ˮ��û�����ʣ�����ˮ�к����Ȼ��ơ��Ȼ��ơ��Ȼ�þ���廯þ�ȴ��������Σ����Լ���ˮ������ˮ�ķ�����
������������Һ������������й����������Ǻ�ˮ������������������ˮ����ѧ�������ֱ��������������Һ���г����������Ǻ�ˮ������������������ˮ��
��2�����õĴ����к��в��������ʣ��ɲ�ȡ���в����ȥ���ȰѴ����ܽ⣬���ù��˵ķ�����ȥ���ʣ�Ȼ������ˮ�֣��õ�������NaCl��
��3����ˮ�е��Ȼ�þ�ܺͼ���Һ��Ӧ����������þ��������ѧ����ʽΪ��MgCl2+Ca��OH��2=CaCl2+Mg��OH��2�����Ӻ�ˮ�й��˷����������������þ������ϡ���ᣬ�Ƶô������Ȼ�þ��Һ����ѧ����ʽΪ��Mg��OH��2+2HCl=MgCl2+2H2O��Ȼ�����Ȼ�þ�����ܵõ�����þ����ѧ����ʽΪ��MgCl2
Mg+Cl2����
�ʴ�Ϊ����1����Һ������������й����������Ǻ�ˮ������������������ˮ���ֱ��������������Һ���г����������Ǻ�ˮ������������������ˮ��
��2�������ᾧ��
��3��MgCl2+Ca��OH��2=CaCl2+Mg��OH��2����MgCl2
Mg+Cl2����
������������Һ������������й����������Ǻ�ˮ������������������ˮ����ѧ�������ֱ��������������Һ���г����������Ǻ�ˮ������������������ˮ��
��2�����õĴ����к��в��������ʣ��ɲ�ȡ���в����ȥ���ȰѴ����ܽ⣬���ù��˵ķ�����ȥ���ʣ�Ȼ������ˮ�֣��õ�������NaCl��
��3����ˮ�е��Ȼ�þ�ܺͼ���Һ��Ӧ����������þ��������ѧ����ʽΪ��MgCl2+Ca��OH��2=CaCl2+Mg��OH��2�����Ӻ�ˮ�й��˷����������������þ������ϡ���ᣬ�Ƶô������Ȼ�þ��Һ����ѧ����ʽΪ��Mg��OH��2+2HCl=MgCl2+2H2O��Ȼ�����Ȼ�þ�����ܵõ�����þ����ѧ����ʽΪ��MgCl2
| ||
�ʴ�Ϊ����1����Һ������������й����������Ǻ�ˮ������������������ˮ���ֱ��������������Һ���г����������Ǻ�ˮ������������������ˮ��
��2�������ᾧ��
��3��MgCl2+Ca��OH��2=CaCl2+Mg��OH��2����MgCl2
| ||

��ϰ��ϵ�д�
�����Ŀ
 �����������ĸ�ף���嫵ĺ�������������ˮ��������̲��ŷḻ�Ļ�ѧ��Դ�����Ȼ��ơ��Ȼ��ơ��Ȼ�þ���廯þ�ȴ��������Σ�
�����������ĸ�ף���嫵ĺ�������������ˮ��������̲��ŷḻ�Ļ�ѧ��Դ�����Ȼ��ơ��Ȼ��ơ��Ȼ�þ���廯þ�ȴ��������Σ� �����������ĸ�ף���嫵ĺ�������������ˮ��������̲��ŷḻ�Ļ�ѧ��Դ�����Ȼ��ơ��Ȼ��ơ��Ȼ�þ���廯þ�ȴ��������Σ�
�����������ĸ�ף���嫵ĺ�������������ˮ��������̲��ŷḻ�Ļ�ѧ��Դ�����Ȼ��ơ��Ȼ��ơ��Ȼ�þ���廯þ�ȴ��������Σ�
