��Ŀ����
��ѧ�Ӻ�ۺ��������Ƕ���ʶ���ʣ���1��������ˮ����ɺͽṹʱ�����Ա���Ϊ��
��ˮ������Ԫ�غ���Ԫ����ɵģ���ˮ���ɴ�����ˮ���Ӿۼ����ɵģ���ÿ1��ˮ��������2����ԭ�Ӻ�1����ԭ�ӹ��ɵģ�
�����������仰�����б�ʾ���ʺ����ɵ���
��2��ͼ�м�������Ԫ����Ԫ�����ڱ��е���Ϣ��ͼ������ԭ�Ӻ������ӵĽṹʾ��ͼ��
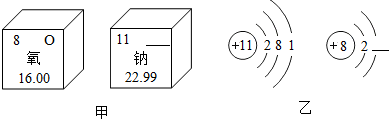
��ͼ�ĺ����������
�ڸ���ͼ�ҵ���Ϣ��д�������ƵĻ�ѧʽ�������Ԫ�صĻ��ϼ�
��3���±����е��Dz���Ԫ�ص��й���Ϣ��
| Ԫ������ | ԭ������������ | ���ӷ��� | �������ϼ� |
| �� | 1 | H+ | +1 |
| þ | 2 | Mg2+ | +2 |
| �� | 3 | Al3+ | +3 |
| �� | 6 | O2- | -2 |
| �� | 7 | Cl- | -1 |
| �� | 6 | S2- | -2 |
��Ԫ�صĻ��ϼ�������������ɵĹ�ϵ��
��Ԫ�صĻ��ϼ��������������Ĺ�ϵ��
��������1�������ɷ��ӹ��ɵ����ʵĻ�ѧʽ�ĺ��壺�ۣ���ʾ���ʵ�һ�����ӣ���ʾһ�����ӵĹ��ɣ���ۣ���ʾһ�����ʣ���ʾ�����ʵ�Ԫ����ɣ������жϣ�
��2���ٸ�����Ԫ�����ڱ��е���Ϣ����֪ͼ�ĺ�����������Ƶ�Ԫ�ط��ţ�
���ݷǽ���Ԫ�صõ������γ������Ϊ8���ȶ����ӽṹ�����н��
�ڸ����ƣ����Ļ��ϼۣ�����ʮ�ֽ��淨��д�������ƵĻ�ѧʽ���ɣ�
��3�����ݱ��еĸ������ݽ��з�����Ѱ�����еĹ��ɣ����н��
��2���ٸ�����Ԫ�����ڱ��е���Ϣ����֪ͼ�ĺ�����������Ƶ�Ԫ�ط��ţ�
���ݷǽ���Ԫ�صõ������γ������Ϊ8���ȶ����ӽṹ�����н��
�ڸ����ƣ����Ļ��ϼۣ�����ʮ�ֽ��淨��д�������ƵĻ�ѧʽ���ɣ�
��3�����ݱ��еĸ������ݽ��з�����Ѱ�����еĹ��ɣ����н��
����⣻��1�������ɷ��ӹ��ɵ����ʵĻ�ѧʽ�ĺ��壺
�ۣ���ʾ���ʵ�һ�����ӣ���ʾһ�����ӵĹ��ɣ�
��ۣ���ʾһ�����ʣ���ʾ�����ʵ�Ԫ����ɣ�
�ɴ˿�֪����ʾ���ʺ����ɵ��� �٢ڣ���ʾ�����۽ṹ���ǣ��ۣ�
�ʴ�Ϊ���٢ڣ� �ۣ�
��2���ٸ�����Ԫ�����ڱ��е���Ϣ����֪ͼ�ĺ�����������Ƶ�Ԫ�ط��ţ���ʾΪ��Na��
���ݷǽ���Ԫ�صõ������γ������Ϊ8���ȶ����ӽṹ������ͼ�Һ����ϵ������� 8��
�ʴ�Ϊ��Na�� 8��
�ڸ����ƣ����Ļ��ϼۣ�����ʮ�ֽ��淨�������ƵĻ�ѧʽΪ��
2
��
�ʴ𰸣�
2
��
��3�����ݱ��еĸ������ݽ��з�����Ѱ�����еĹ��ɣ�
��Ԫ�صĻ��ϼ�������������ɵĹ�ϵ�ǣ���ֵ��ȣ���ʾ������ͬ��
�ʴ�Ϊ����ֵ��ȣ���ʾ������ͬ��
��Ԫ�صĻ��ϼ��������������Ĺ�ϵ�ǣ��������������ԭ���������Ӹ���������������8��ȥ�������Ӹ�����
�ʴ�Ϊ���������������ԭ���������Ӹ���������������8��ȥ�������Ӹ�����
�ۣ���ʾ���ʵ�һ�����ӣ���ʾһ�����ӵĹ��ɣ�
��ۣ���ʾһ�����ʣ���ʾ�����ʵ�Ԫ����ɣ�
�ɴ˿�֪����ʾ���ʺ����ɵ��� �٢ڣ���ʾ�����۽ṹ���ǣ��ۣ�
�ʴ�Ϊ���٢ڣ� �ۣ�
��2���ٸ�����Ԫ�����ڱ��е���Ϣ����֪ͼ�ĺ�����������Ƶ�Ԫ�ط��ţ���ʾΪ��Na��
���ݷǽ���Ԫ�صõ������γ������Ϊ8���ȶ����ӽṹ������ͼ�Һ����ϵ������� 8��
�ʴ�Ϊ��Na�� 8��
�ڸ����ƣ����Ļ��ϼۣ�����ʮ�ֽ��淨�������ƵĻ�ѧʽΪ��
| +1 |
| Na |
| -2 |
| O |
�ʴ𰸣�
| +1 |
| Na |
| -2 |
| O |
��3�����ݱ��еĸ������ݽ��з�����Ѱ�����еĹ��ɣ�
��Ԫ�صĻ��ϼ�������������ɵĹ�ϵ�ǣ���ֵ��ȣ���ʾ������ͬ��
�ʴ�Ϊ����ֵ��ȣ���ʾ������ͬ��
��Ԫ�صĻ��ϼ��������������Ĺ�ϵ�ǣ��������������ԭ���������Ӹ���������������8��ȥ�������Ӹ�����
�ʴ�Ϊ���������������ԭ���������Ӹ���������������8��ȥ�������Ӹ�����
���������⿼��ѧ����������е���Ϣ�������ۺϷ����ܽ���ɣ������������

��ϰ��ϵ�д�
 ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�
�����Ŀ
��ѧ�Ӻ�ۺ��������Ƕ���ʶ���ʣ�
��1��������ˮ����ɺͽṹʱ�����Ա���Ϊ��
��ˮ������Ԫ�غ���Ԫ����ɵģ���ˮ���ɴ�����ˮ���Ӿۼ����ɵģ���ÿ1��ˮ��������2����ԭ�Ӻ�1����ԭ�ӹ��ɵģ�
�����������仰�����б�ʾ���ʺ����ɵ��� ����ʾ�����۽ṹ���� ��������ţ�
��2��ͼ�м�������Ԫ����Ԫ�����ڱ��е���Ϣ��ͼ������ԭ�Ӻ������ӵĽṹʾ��ͼ��
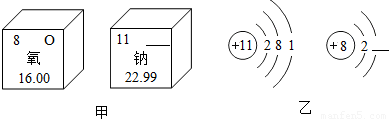
��ͼ�ĺ���������� ��ͼ�Һ����ϵ������� ��
�ڸ���ͼ�ҵ���Ϣ��д�������ƵĻ�ѧʽ�������Ԫ�صĻ��ϼ� ��
��3���±����е��Dz���Ԫ�ص��й���Ϣ��
����Ա��еĸ������ݽ��з�����Ѱ�����еĹ��ɣ����ܵó��Ľ����ǣ�
��Ԫ�صĻ��ϼ�������������ɵĹ�ϵ�� ��
��Ԫ�صĻ��ϼ��������������Ĺ�ϵ�� ��
��1��������ˮ����ɺͽṹʱ�����Ա���Ϊ��
��ˮ������Ԫ�غ���Ԫ����ɵģ���ˮ���ɴ�����ˮ���Ӿۼ����ɵģ���ÿ1��ˮ��������2����ԭ�Ӻ�1����ԭ�ӹ��ɵģ�
�����������仰�����б�ʾ���ʺ����ɵ��� ����ʾ�����۽ṹ���� ��������ţ�
��2��ͼ�м�������Ԫ����Ԫ�����ڱ��е���Ϣ��ͼ������ԭ�Ӻ������ӵĽṹʾ��ͼ��

��ͼ�ĺ���������� ��ͼ�Һ����ϵ������� ��
�ڸ���ͼ�ҵ���Ϣ��д�������ƵĻ�ѧʽ�������Ԫ�صĻ��ϼ� ��
��3���±����е��Dz���Ԫ�ص��й���Ϣ��
| Ԫ������ | ԭ������������ | ���ӷ��� | �������ϼ� |
| �� | 1 | H+ | +1 |
| þ | 2 | Mg2+ | +2 |
| �� | 3 | Al3+ | +3 |
| �� | 6 | O2- | -2 |
| �� | 7 | Cl- | -1 |
| �� | 6 | S2- | -2 |
��Ԫ�صĻ��ϼ�������������ɵĹ�ϵ�� ��
��Ԫ�صĻ��ϼ��������������Ĺ�ϵ�� ��
��ѧ�Ӻ�ۺ��������Ƕ���ʶ���ʣ�
��1��������ˮ����ɺͽṹʱ�����Ա���Ϊ��
��ˮ������Ԫ�غ���Ԫ����ɵģ���ˮ���ɴ�����ˮ���Ӿۼ����ɵģ���ÿ1��ˮ��������2����ԭ�Ӻ�1����ԭ�ӹ��ɵģ�
�����������仰�����б�ʾ���ʺ����ɵ��� ����ʾ�����۽ṹ���� ��������ţ�
��2��ͼ�м�������Ԫ����Ԫ�����ڱ��е���Ϣ��ͼ������ԭ�Ӻ������ӵĽṹʾ��ͼ��

��ͼ�ĺ���������� ��ͼ�Һ����ϵ������� ��
�ڸ���ͼ�ҵ���Ϣ��д�������ƵĻ�ѧʽ�������Ԫ�صĻ��ϼ� ��
��3���±����е��Dz���Ԫ�ص��й���Ϣ��
����Ա��еĸ������ݽ��з�����Ѱ�����еĹ��ɣ����ܵó��Ľ����ǣ�
��Ԫ�صĻ��ϼ�������������ɵĹ�ϵ�� ��
��Ԫ�صĻ��ϼ��������������Ĺ�ϵ�� ��
��1��������ˮ����ɺͽṹʱ�����Ա���Ϊ��
��ˮ������Ԫ�غ���Ԫ����ɵģ���ˮ���ɴ�����ˮ���Ӿۼ����ɵģ���ÿ1��ˮ��������2����ԭ�Ӻ�1����ԭ�ӹ��ɵģ�
�����������仰�����б�ʾ���ʺ����ɵ��� ����ʾ�����۽ṹ���� ��������ţ�
��2��ͼ�м�������Ԫ����Ԫ�����ڱ��е���Ϣ��ͼ������ԭ�Ӻ������ӵĽṹʾ��ͼ��

��ͼ�ĺ���������� ��ͼ�Һ����ϵ������� ��
�ڸ���ͼ�ҵ���Ϣ��д�������ƵĻ�ѧʽ�������Ԫ�صĻ��ϼ� ��
��3���±����е��Dz���Ԫ�ص��й���Ϣ��
| Ԫ������ | ԭ������������ | ���ӷ��� | �������ϼ� |
| �� | 1 | H+ | +1 |
| þ | 2 | Mg2+ | +2 |
| �� | 3 | Al3+ | +3 |
| �� | 6 | O2- | -2 |
| �� | 7 | Cl- | -1 |
| �� | 6 | S2- | -2 |
��Ԫ�صĻ��ϼ�������������ɵĹ�ϵ�� ��
��Ԫ�صĻ��ϼ��������������Ĺ�ϵ�� ��
��ѧ�Ӻ�ۺ��������Ƕ���ʶ���ʣ�
��1��������ˮ����ɺͽṹʱ�����Ա���Ϊ��
��ˮ������Ԫ�غ���Ԫ����ɵģ���ˮ���ɴ�����ˮ���Ӿۼ����ɵģ���ÿ1��ˮ��������2����ԭ�Ӻ�1����ԭ�ӹ��ɵģ�
�����������仰�����б�ʾ���ʺ����ɵ��� ����ʾ�����۽ṹ���� ��������ţ�
��2��ͼ�м�������Ԫ����Ԫ�����ڱ��е���Ϣ��ͼ������ԭ�Ӻ������ӵĽṹʾ��ͼ��

��ͼ�ĺ���������� ��ͼ�Һ����ϵ������� ��
�ڸ���ͼ�ҵ���Ϣ��д�������ƵĻ�ѧʽ�������Ԫ�صĻ��ϼ� ��
��3���±����е��Dz���Ԫ�ص��й���Ϣ��
����Ա��еĸ������ݽ��з�����Ѱ�����еĹ��ɣ����ܵó��Ľ����ǣ�
��Ԫ�صĻ��ϼ�������������ɵĹ�ϵ�� ��
��Ԫ�صĻ��ϼ��������������Ĺ�ϵ�� ��
��1��������ˮ����ɺͽṹʱ�����Ա���Ϊ��
��ˮ������Ԫ�غ���Ԫ����ɵģ���ˮ���ɴ�����ˮ���Ӿۼ����ɵģ���ÿ1��ˮ��������2����ԭ�Ӻ�1����ԭ�ӹ��ɵģ�
�����������仰�����б�ʾ���ʺ����ɵ��� ����ʾ�����۽ṹ���� ��������ţ�
��2��ͼ�м�������Ԫ����Ԫ�����ڱ��е���Ϣ��ͼ������ԭ�Ӻ������ӵĽṹʾ��ͼ��

��ͼ�ĺ���������� ��ͼ�Һ����ϵ������� ��
�ڸ���ͼ�ҵ���Ϣ��д�������ƵĻ�ѧʽ�������Ԫ�صĻ��ϼ� ��
��3���±����е��Dz���Ԫ�ص��й���Ϣ��
| Ԫ������ | ԭ������������ | ���ӷ��� | �������ϼ� |
| �� | 1 | H+ | +1 |
| þ | 2 | Mg2+ | +2 |
| �� | 3 | Al3+ | +3 |
| �� | 6 | O2- | -2 |
| �� | 7 | Cl- | -1 |
| �� | 6 | S2- | -2 |
��Ԫ�صĻ��ϼ�������������ɵĹ�ϵ�� ��
��Ԫ�صĻ��ϼ��������������Ĺ�ϵ�� ��
