��Ŀ����
ͼ1��A��B���ֹ������ʵ��ܽ�����ߣ�ͼ2��ʾ�IJ����ǣ�20��ʱ���ֱ���100gˮ�м���������ļס��ҹ��壬����ܽ���������ͼ�ش��й����⡣

��1�������Ҷ�Ӧ���ܽ��������_______���A����B����.
��2��50��ʱ����A�ı�����Һ��һ��������ˮ���Ƴ�30%��A��Һ100.0g������ˮ������Ϊ________��
��3��ͼ1�У�A��Һ��m�㵽p��ľ��巽����________��
��4������˵����ȷ����________
A 40��ʱ��A��B�ı�����Һ�����ʵ������������
B 50��ʱ������A��B�ı�����Һ���µ�40��ʱ����Һ��������С��ϵ�� A��B
C��ͼ2�����ձ��ڵ��������µ�50��ʱ��������Һһ���Dz�������Һ�����ʡ���Һ�����������䣨������ˮ��������
D ���ҵı�����Һ�к������ף�����������ܼ��ᾧ�ķ����ᴿ�ң�Ҳ������ȴ�ȱ�����Һ�ᾧ�ķ���
 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д��������и����е��������ʣ���ѡ�Լ�����Ʒ�ܴﵽĿ����(����)
ѡ�� | ���������� | ��ѡ�Լ�����Ʒ |
A | NaOH��Һ��ϡHCl��ϡH2SO4 | ��ɫʯ����Һ |
B | NaOH��Һ��NaCl��Һ��ʳ�� | ��̪��Һ |
C | NaOH���塢NaCl���塢NH4NO3���� | ˮ |
D | ����ˮ��NaCl��Һ��Na2CO3��Һ | pH��ֽ |
A. A B. B C. C D. D




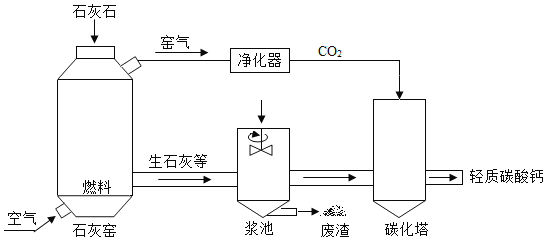
 CaO+CO2��,��Ҫ�Ƶ�56 t �����ƣ���������Ҫ��̼�������Ϊ______t��
CaO+CO2��,��Ҫ�Ƶ�56 t �����ƣ���������Ҫ��̼�������Ϊ______t��