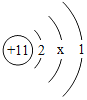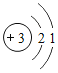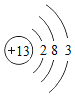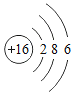��Ŀ����
����Ŀ����ѧʹ��������ã�ͨ��ѧϰ�������кܶ���ջ�����ѧ���˴Ӻ�ۺ��۵ĽǶ���ʶ�������硣
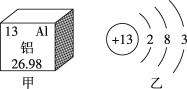
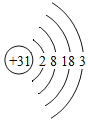
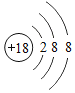
��1�����𡱺͡���ʾ��ͬԪ�ص�ԭ�ӣ�����ͼʾ��ʾ��������_____��ѡ���ţ���
A  B
B  C
C 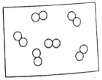 D
D 
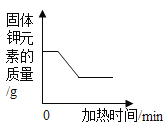
��2����ͼ��ij�ܱ����������ʱ仯���̵���ʾ��ͼ������ʾ��ԭ�ӣ����𡱱�ʾ��ԭ�ӣ���

��ش�
��д���仯I�Ļ�ѧ����ʽ��_____��
�ڱ仯I�Ĺ����У�û�з����仯����С����_____���������ƣ���
�����й��ڱ仯II��˵���У���ȷ����_____����д��ţ���
A ���ӵĻ�ѧ����û�иı�
B ���ӱ�С��
C ���Ӽ�ľ����С��
D ���ӵ�������˸ı�
��3��ʳƷ�г���������������������ƣ�C6H5COONa�����÷�������_____��Ԫ����ɡ�Ħ������Ϊ_____��0.5 mol�ı���������Լ����_____����ԭ�ӡ�
��4��������顱�롰Ư�������ܻ��ã�������ײ���һ���ж�����X����Ӧԭ��Ϊ��NaClO+2HCl��NaCl+X��+H2O����X�Ļ�ѧʽΪ_____����Ӧ����Ԫ�صĴ�����̬Ϊ_____�����ţ���
A ����̬ B ����̬ C ��������̬���л���̬
���𰸡�AD ![]() ��ԭ�Ӻ���ԭ�� AC �� 144g/mol
��ԭ�Ӻ���ԭ�� AC �� 144g/mol ![]() Cl2 C
Cl2 C
��������
����ͼʾ��Ϣ��������ѧ�仯�ı��ʸ�����з������
��1���������۵ĽǶȷ���Ϊ���ж��ֽṹ�����ʣ��۲�ͼʾ��Ϣ��֪A��D�о��������ֽṹ�����ڻ����ʱ�ʾ��������AD��
��2������ͼʾ��Ϣ��֪�仯IΪ�����������ڵ�ȼ������������ˮ�����䷴Ӧ����ʽΪ![]() ��
��
����ѧ�仯����С������Ϊԭ�ӣ��ʱ仯I�Ĺ����У�û�з����仯����С������ԭ�Ӻ���ԭ�ӣ�
���۲�ͼʾ��Ϣ�б仯II��֪��仯����Ϊ��ȴ�����£�����û�����������ɣ�Ϊ�����仯����Ϊ�������仯�ʷ�������䣬���ӵĻ�ѧ����Ҳ���䣬��A ��ȷ��D����ȷ����ȴ������ֻ�Ƿ��Ӽ�����С��������С����ı䣬��B����ȷ��C��ȷ��
��ѡAC��
��3��ʳƷ�г���������������������ƣ�C6H5COONa����C6H5COONa�к���C��H��O��Na������Ԫ�أ��ʸ÷�����������Ԫ����ɡ�Ħ����������ֵ��������Է���������ͬ���䵥λΪg/mol����C6H5COONa��Է�������Ϊ![]() ������Ħ������Ϊ144g/mol��1mol���ĸ�����ԼΪ
������Ħ������Ϊ144g/mol��1mol���ĸ�����ԼΪ![]() ������һ��C6H5COONa�����к���2����ԭ�ӣ��ʣ�0.5 mol�ı���������Լ������ԭ�Ӹ���Ϊ
������һ��C6H5COONa�����к���2����ԭ�ӣ��ʣ�0.5 mol�ı���������Լ������ԭ�Ӹ���Ϊ![]() ����
����
��4�����ݻ�ѧ�仯��ԭ�Ӹ����غ��֪NaClO+2HCl��NaCl+X��+H2O��X����2����ԭ�ӣ���X��ѧʽΪCl2����Ӧ���NaCl���ڻ���̬����Cl2��������̬����������̬���л���̬����ѡC��