��Ŀ����
28���κο�ѧ���۶�Ҫ����ʵ����֤��ʵ������Ҫ��ѧ��������
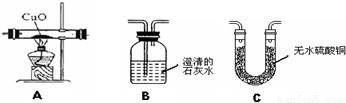
ΪŪ���ij�����ķ��ն�������ɷ����Ƿ���ˮ������һ����̼�Ͷ�����̼���ס�������ʵ��С��ֱ�������ʢ��ҩƷ��������ͼ�мг������Ⱦ�ʡ�ԣ���������ն�������ɷ֣�������B��C��D������ȫ��

�ټ�����һ�����Ƿ�������ijһ�����壬�����������Ƿ��ж�����̼��Ӧѡ�ã����ţ�
������������������װһ��װ�ã�ͨ��һ��ʵ��ͬʱ�����������壬������ͨ�����Ⱥ�˳�����ӵ����������ǣ����ţ����������ظ�ʹ�ã�
��������֤���ն��������в�����һ����̼�������ǽ����ն�ǰ��Ҫ���ж�����̼�����Ƿ�ϸߵ�ʵ�飬�䷽����
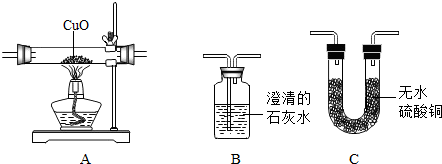
ΪŪ���ij�����ķ��ն�������ɷ����Ƿ���ˮ������һ����̼�Ͷ�����̼���ס�������ʵ��С��ֱ�������ʢ��ҩƷ��������ͼ�мг������Ⱦ�ʡ�ԣ���������ն�������ɷ֣�������B��C��D������ȫ��

�ټ�����һ�����Ƿ�������ijһ�����壬�����������Ƿ��ж�����̼��Ӧѡ�ã����ţ�
D
�������ն��ں���ˮ��������B�е���������ɫ�����ɫ
����A�в������������ɺ�ɫ��ɺ�ɫ������ն��ں���CO
��������������������װһ��װ�ã�ͨ��һ��ʵ��ͬʱ�����������壬������ͨ�����Ⱥ�˳�����ӵ����������ǣ����ţ����������ظ�ʹ�ã�
BDCA
����������֤���ն��������в�����һ����̼�������ǽ����ն�ǰ��Ҫ���ж�����̼�����Ƿ�ϸߵ�ʵ�飬�䷽����
��ȼ��������ѣ����Ƿ�Ϩ��
�����ܰ�ȫ���붴�У������������dz�������ļ��鷽����ˮ����һ������ˮ����ͭ���飬��ɫ�����ɫ��һ����̼һ���ü��ȵ�����ͭ���飻������̼һ���ó����ʯ��ˮ���飬������������Һ���գ���������ʱ��Ϊ������ţ�Ҫע���Ⱥ�˳��һ����ˮ����ʱ���ȼ���ˮ�������ڼ����������壮����Ƕ�������и��Ӧ�������и�����������̼�������ߣ�Ҫ������ն��ͱ������ƻ�ʵ�飮
����⣺�ٶ�����̼һ���ó����ʯ��ˮ���飬���ѡװ��D����ɫ����ˮ����ͭ��ˮ����ɫ��A�в������������ɺ�ɫ��ɺ�ɫ������Ϊһ����̼���˻�ԭ�����ʴ�Ϊ��D����ɫ�����ɫ��CO��
����ˮ������һ����̼�Ͷ�����̼��Ҫ�ȼ���ˮ�������ټ��������̼���Ѷ�����̼����֮���ټ���һ����̼���ʴ�Ϊ��BDCA��
�۽����ն�ǰ��Ҫ���ж�����̼�����Ƿ�ϸߵ�ʵ�飬��Ҫ���ƻ�ʵ�飮�ʴ�Ϊ����ȼ��������ѣ����Ƿ�Ϩ��
����ˮ������һ����̼�Ͷ�����̼��Ҫ�ȼ���ˮ�������ټ��������̼���Ѷ�����̼����֮���ټ���һ����̼���ʴ�Ϊ��BDCA��
�۽����ն�ǰ��Ҫ���ж�����̼�����Ƿ�ϸߵ�ʵ�飬��Ҫ���ƻ�ʵ�飮�ʴ�Ϊ����ȼ��������ѣ����Ƿ�Ϩ��
�����������㿼���˳�������ļ���ͳ��ӷ�����Ҫ��ͬѧ�Dz���Ҫ������������ʣ���Ҫ�˽������Լ������ʣ���ʵ�������Ϊ������ţ�Ҫע������ʵ��Ⱥ�˳��Ȼ���ۺϷ���������Ӧ�ã��𰸾ͻ�ӭ�ж��⣮��������Ҫ������ʵ�����У�

��ϰ��ϵ�д�
�����Ŀ
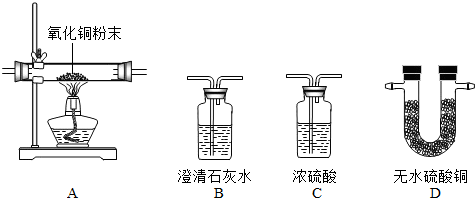
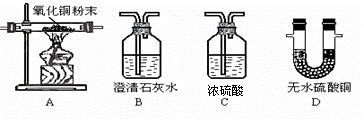 �κο�ѧ���۶�Ҫ����ʵ����֤��ʵ������Ҫ��ѧ��������ΪŪ���ij�����ķ��ն�������ɷ����Ƿ���ˮ������������һ����̼�Ͷ�����̼���ס�������ʵ��С��ֱ�������ʢ��ҩƷ��������ͼ�мг������Ⱦ�ʡ�ԣ���������ն�������ɷ֡�������B��C��D������ȫ��
�κο�ѧ���۶�Ҫ����ʵ����֤��ʵ������Ҫ��ѧ��������ΪŪ���ij�����ķ��ն�������ɷ����Ƿ���ˮ������������һ����̼�Ͷ�����̼���ס�������ʵ��С��ֱ�������ʢ��ҩƷ��������ͼ�мг������Ⱦ�ʡ�ԣ���������ն�������ɷ֡�������B��C��D������ȫ�� ___________��
___________��