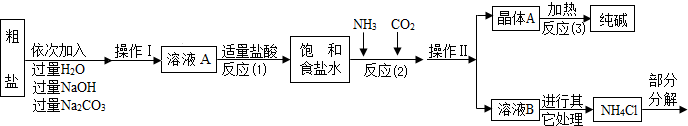
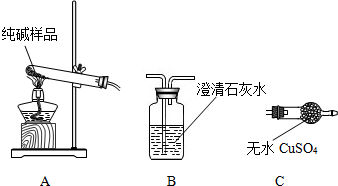
��Ŀ����
��1����ͼ��ʾ��ij������庬�г��л�ѧ���������壬��������ͨ��װ�г����ʯ��ˮ�ļ��������Լ�ƿ�����������������еij����ʯ��ˮ����ǡ��������ijɷ�Ϊ___��___�����з�����Ӧ�Ļ�ѧ����ʽΪ___��___��
| | A | B | C | D |
| A | �� | �� | �� | �� |
| B | �� | �� | �� | �� |
| C | �� | �� | �� | �� |
| D | �� | �� | �� | �� |
��BΪ___��___���ѧʽ����
��A��C������Ӧ�Ļ�ѧ����ʽΪ___��___���÷�Ӧ�Ļ�����Ӧ������___��___��
��3��ij����ѧϰС����̼������Һ�ֱ�������ʯ��ˮ�����ᡣʵ���Һ����ͬһ�ձ��У�����ձ���Һ����塣��ʦ��������С���ͬѧ���۷�������Ϊ���ձ��г������Һ���ܻẬ���������ʣ�Na2CO3��HCl��Ca(OH)2��NaOH��NaCl��CaCl2��������ݳ�����ѧ��ѧ֪ʶ�жϣ����ձ�������Һ��һ�����е�������___��___��һ�������е�������___��___��
��1��__HCl��CO2__�� Ca(OH)2+2HCl=CaCl2+2H2O ��
��2���� HCl ���� Na2CO3+Ca(OH)2=CaCO3��+2NaOH �����ֽⷴӦ ��
��3�� NaCl��CaCl2 �� Ca(OH)2��Na2CO3��NaOH ������:
��
��2���� HCl ���� Na2CO3+Ca(OH)2=CaCO3��+2NaOH �����ֽⷴӦ ��
��3�� NaCl��CaCl2 �� Ca(OH)2��Na2CO3��NaOH ������:
��

��ϰ��ϵ�д�
 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д�
�����Ŀ