��Ŀ����
��ͼ��ʵ���ҳ��õ�װ�á����ͼ�ش�
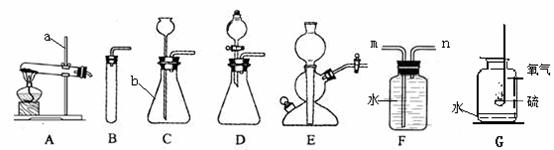
�������a������ ������b������ ��
��д����װ��A��ȡ�����Ļ�ѧ![]() ����ʽ�� ���÷�Ӧ�Ļ��������� ������װ��F�ռ������Բ���ռ�O2����������� ����m��n���˽�һ����Ͳ�Ա����ų���ˮ�������
����ʽ�� ���÷�Ӧ�Ļ��������� ������װ��F�ռ������Բ���ռ�O2����������� ����m��n���˽�һ����Ͳ�Ա����ų���ˮ�������
��Gͼ������������ȼ�յ�ʵ�飬�۲쵽�������� ���Ը�ʵ������˸Ľ����ڼ���ƿ�����ȼ�������ˮ�������ú�Ŀ���� ��
�������Ʊ�CO2��ѡ�õķ���װ���У� (��д��ͼ��ĸ���)���Ƚ�B��Eװ�ã�Eװ�õ��ŵ�Ϊ�� ��
������������ʯ��ʯ��100��ϡ��������ȫ��Ӧ![]() ���Ƶö�����̼Ϊ8.8�ˡ�
���Ƶö�����̼Ϊ8.8�ˡ�
�Լ�![]() �㣺
�㣺
��100��ϡ����������HCl�����ʵ��������ݻ�ѧ����ʽ��ʽ���㣩��
�ڸ�ϡ������HCl����������Ϊ ��
������̨����ƿ
��2KClO3![]() 2KCl+3O2�����ֽⷴӦ��m.
2KCl+3O2�����ֽⷴӦ��m.
�Ƿ�������������ɫ���棬�����̼�����ζ�����壬�ų�![]() ���������ն�������ֹ���ɶ���������й��Ⱦ������
���������ն�������ֹ���ɶ���������й��Ⱦ������
��BCDE(��ȫ��1��)��
����ʱ��Һ�巴Ӧ�������Һ���Լ������ᣩ���㣨1�֣�������ʹ��Ӧ��ʱ������ֹͣ����ԼҩƷ��1�֣�����2�֣�
�ɢ�CO2�����ʵ���=8.8��/44��/Ħ��=0.2Ħ��
�裺��HClΪXĦ��
CaCO3+2HCl��CaCl2+CO2��+H![]() 2O
2O
2 1
X 0.2
2/ X =1/0.2
X=0.4��Ħ����
��14.6%��0.146

 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�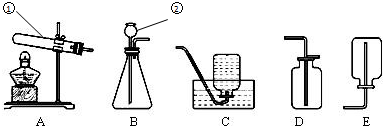
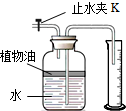 K��Ȼ������ë����ס����ƿ��
K��Ȼ������ë����ס����ƿ��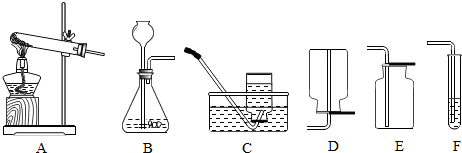


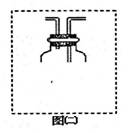
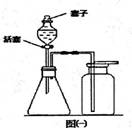
 ��ͼ��ʵ���ҳ��õ�װ�á����ͼ�ش�
��ͼ��ʵ���ҳ��õ�װ�á����ͼ�ش�