��Ŀ����
ͨ��һ��Ļ�ѧѧϰ��������ʶ�ˡ��ᡱ����������Ρ�����ش��������⣺
��1���������������ᡢ����ȣ������ǵ�ˮ��Һ�����ڴ�������ͬ��________������ţ�����ˣ������кܶ����ƵĻ�ѧ���ʣ�
��2�������ļ����������ơ��������Ƶȣ��������ƿ�����ʯ����ˮ��Ӧ�Ƶã���Ӧ�Ļ�ѧ����ʽΪ_____________________________________��
��3��̼��������һ�ֳ������Σ�������θ�����֢�����˷��ú���θ�ᷴӦ���÷�Ӧ�Ļ�ѧ����ʽΪ_______________________________________________��
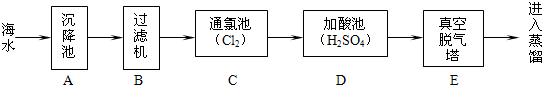
��4���кͷ�Ӧ���ճ������ũҵ�������й㷺��Ӧ�ã������᳧����ˮ�к�����������ʣ�������ʯ�ҽ��д�������Ӧ�Ļ�ѧ����ʽΪ__________________________________��
��1���������������ᡢ����ȣ������ǵ�ˮ��Һ�����ڴ�������ͬ��________������ţ�����ˣ������кܶ����ƵĻ�ѧ���ʣ�
��2�������ļ����������ơ��������Ƶȣ��������ƿ�����ʯ����ˮ��Ӧ�Ƶã���Ӧ�Ļ�ѧ����ʽΪ_____________________________________��
��3��̼��������һ�ֳ������Σ�������θ�����֢�����˷��ú���θ�ᷴӦ���÷�Ӧ�Ļ�ѧ����ʽΪ_______________________________________________��
��4���кͷ�Ӧ���ճ������ũҵ�������й㷺��Ӧ�ã������᳧����ˮ�к�����������ʣ�������ʯ�ҽ��д�������Ӧ�Ļ�ѧ����ʽΪ__________________________________��
��1��H+ ��2��CaO + H2O��Ca(OH)2 ��3��NaHCO3 + HCl��NaCl + H2O + CO2��
��4��H2SO4 + Ca(OH)2��CaSO4 + 2H2O
��4��H2SO4 + Ca(OH)2��CaSO4 + 2H2O
���������(1)�������������ᡢ����ȣ������ǵ�ˮ��Һ�����ڴ�������ͬ��H+�������кܶ����ƵĻ�ѧ����
(2)��ʯ����ˮ��Ӧ�Ļ�ѧ����ʽΪ��CaO + H2O��Ca(OH)2
(3)θ�����Ҫ�ɷ������ᣬ��̼�����Ʒ�Ӧ�Ļ�ѧ����ʽΪ��NaHCO3 + HCl��NaCl + H2O + CO2��
��4����������ʯ�ҷ�Ӧ�Ļ�ѧ����ʽΪ��H2SO4 + Ca(OH)2��CaSO4 + 2H2O

��ϰ��ϵ�д�
�����Ŀ