��Ŀ����
��ͭ����ͭ��п�γɵĺϽ��н�ǿ����ĥ���ܡ������Ժá��ӹ������������й㷺����;��������������������DZ��ȣ�ijͬѧΪ�˲ⶨij��ͭм��Ʒ����ɣ�������ȡ��Ʒ��ϡ���ᷴӦ����ʵ�����ݼ�¼���±���
���㣺
��1��ͨ�������������ݿ�֪������ͭ��Ʒ��ϡ�����������Ϊ
��2�����ͭ�Ͻ���ͭ������������
��3��������ϡ���������������������
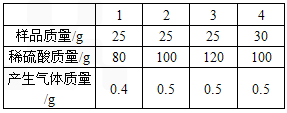
| 1 | 2 | 3 | 4 | |
| ��Ʒ����/g | 25 | 25 | 25 | 30 |
| ϡ��������/g | 80 | 100 | 120 | 100 |
| ������������/g | 0.4 | 0.5 | 0.5 | 0.5 |
��1��ͨ�������������ݿ�֪������ͭ��Ʒ��ϡ�����������Ϊ
1��4
1��4
ʱ������ǡ����ȫ��Ӧ����2�����ͭ�Ͻ���ͭ������������
��3��������ϡ���������������������
��������1�����Ĵη�Ӧ�����ݿ�֪�ڶ���������ȫ��Ӧ������
��2���ȸ���п��ϡ���ᷴӦ�Ļ�ѧ����ʽ���25g��ͭ��п��������25g��ȥп����������ͭ���������ٳ���25g������⣮
��3�����ݵڶ���ʵ���������������������100gϡ�����������������������100g����100%���ɷ�����
��2���ȸ���п��ϡ���ᷴӦ�Ļ�ѧ����ʽ���25g��ͭ��п��������25g��ȥп����������ͭ���������ٳ���25g������⣮
��3�����ݵڶ���ʵ���������������������100gϡ�����������������������100g����100%���ɷ�����
����⣺��1�����Ĵη�Ӧ�����ݿ�֪�ڶ���������ȫ��Ӧ���ʴ𰸣�1��4��
��2����25g��Ʒ��п������Ϊx��100gϡ���������ʵ�����Ϊy��
Zn+H2SO4=ZnSO4+H2��
65 98 2
x y 0.5g
=
x=16.25g
=
y=24.5g
25g��ͭ��ͭ������Ϊ25g-16.25g=8.75g
��ͭ�Ͻ���ͭ����������Ϊ��
=35%
�𣺻�ͭ�Ͻ���ͭ����������Ϊ35%��
��3��ϡ������������������Ϊ
��100%=24.5%
������ϡ�������������������Ϊ24.5%
��2����25g��Ʒ��п������Ϊx��100gϡ���������ʵ�����Ϊy��
Zn+H2SO4=ZnSO4+H2��
65 98 2
x y 0.5g
| 65 |
| x |
| 2 |
| 0.5g |
x=16.25g
| 98 |
| y |
| 2 |
| 0.5g |
y=24.5g
25g��ͭ��ͭ������Ϊ25g-16.25g=8.75g
��ͭ�Ͻ���ͭ����������Ϊ��
| 8.75g |
| 25g |
�𣺻�ͭ�Ͻ���ͭ����������Ϊ35%��
��3��ϡ������������������Ϊ
| 24.5g |
| 100g |
������ϡ�������������������Ϊ24.5%
�����������Ƿ���ʵ�����ݵĻ�ѧ����ʽ�ļ��㣬�ؼ��Ǵӱ������ݷ������ʲôʱ��ǡ����ȫ��Ӧ����һ���Ѷȣ�

��ϰ��ϵ�д�
�����Ŀ
��ͭ����ͭ��п�γɵĺϽ��н�ǿ����ĥ���ܡ������Ժá��ӹ������������й㷺����;��������������������DZ��ȣ�ijͬѧΪ�˲ⶨij��ͭм��Ʒ����ɣ�������ȡ��Ʒ��ϡ���ᷴӦ����ʵ�����ݼ�¼���±���
| 1 | 2 | 3 | 4 | |
| ��Ʒ����/g | 25 | 25 | 25 | 30 |
| ϡ��������/g | 80 | 100 | 120 | 100 |
| ������������/g | 0.4 | 0.5 | 0.5 | 0.5 |
��1��ͨ�������������ݿ�֪������ͭ��Ʒ��ϡ�����������Ϊ________ʱ������ǡ����ȫ��Ӧ��
��2�����ͭ�Ͻ���ͭ������������
��3��������ϡ���������������������
��ͭ����ͭ��п�γɵĺϽ��н�ǿ����ĥ���ܡ������Ժá��ӹ������������й㷺����;��������������������DZ��ȣ�ijͬѧΪ�˲ⶨij��ͭм��Ʒ����ɣ�������ȡ��Ʒ��ϡ���ᷴӦ����ʵ�����ݼ�¼���±���
���㣺
��1��ͨ�������������ݿ�֪������ͭ��Ʒ��ϡ�����������Ϊ______ʱ������ǡ����ȫ��Ӧ��
��2�����ͭ�Ͻ���ͭ������������
��3��������ϡ���������������������
| 1 | 2 | 3 | 4 | |
| ��Ʒ����/g | 25 | 25 | 25 | 30 |
| ϡ��������/g | 80 | 100 | 120 | 100 |
| ������������/g | 0.4 | 0.5 | 0.5 | 0.5 |
��1��ͨ�������������ݿ�֪������ͭ��Ʒ��ϡ�����������Ϊ______ʱ������ǡ����ȫ��Ӧ��
��2�����ͭ�Ͻ���ͭ������������
��3��������ϡ���������������������
��ͭ����ͭ��п�γɵĺϽ��н�ǿ����ĥ���ܡ������Ժá��ӹ������������й㷺����;��������������������DZ��ȣ�ijͬѧΪ�˲ⶨij��ͭм��Ʒ����ɣ�������ȡ��Ʒ��ϡ���ᷴӦ����ʵ�����ݼ�¼���±���
���㣺
��1��ͨ�������������ݿ�֪������ͭ��Ʒ��ϡ�����������Ϊ______ʱ������ǡ����ȫ��Ӧ��
��2�����ͭ�Ͻ���ͭ������������
��3��������ϡ���������������������
| 1 | 2 | 3 | 4 | |
| ��Ʒ����/g | 25 | 25 | 25 | 30 |
| ϡ��������/g | 80 | 100 | 120 | 100 |
| ������������/g | 0.4 | 0.5 | 0.5 | 0.5 |
��1��ͨ�������������ݿ�֪������ͭ��Ʒ��ϡ�����������Ϊ______ʱ������ǡ����ȫ��Ӧ��
��2�����ͭ�Ͻ���ͭ������������
��3��������ϡ���������������������