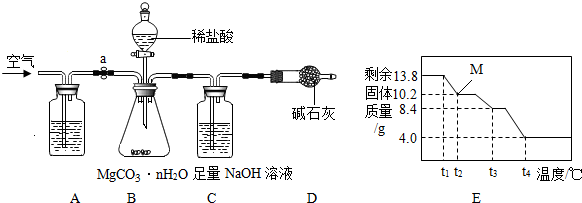
��Ŀ����

[��������]��
a��̼��þ�������ȷֽ⣬����3�������
b����Է���������[Mr��MgCO3����84��Mr��H2O����18��Mr��MgO����40��Mr��CO2����44]
[ʵ�鲽��]
��
��ȷ��ȡ3.45g MgCO3?nH2O����Bװ���У�����������
�۴��ɼ�a������һ��ʱ�����������Cװ�õ�������
�ܹرյ��ɼ�a����Һ©����������������ϡ���������ٲ�������Ϊֹ��
�ݴ��ɼ�a��
��ȷ����Cװ�õ��������������
| �� �� | 1 | 2 | 3 |
| ʵ��ǰ | 228.00 | 228.00 | 228.00 |
| ʵ��� | 229.10 | 229.11 | 229.09 |
��������ݽ��м��㣮
[ʵ����������ݴ���]
��1������ʵ�鲽�裺��
��2�������̼��þ�����е�nֵ����Ҫ���м�����̣�3�֣�
��3��Aװ����ʢ�ŵ���Һ��
��4��Dװ�õ�������
[ʵ�鷴˼]
��5��ʵ����������ֵ��ƫ�ԭ�������
��6������ѡ���У������ʵ����ƫ�����
�ٷ�Ӧ������û��ͨ������ �ڳ���Cװ��ǰ��û��ͨ������
��û��Aװ�ã� ��û��Dװ�ã�
��ϡ����μ�����̫�죻 ��Cװ����NaOH��ҺŨ�ȹ���
��7��Ϊ��ȷ�ⶨn��ֵ������ʦָ���£�С��ͬѧ��ȡ13.8g MgCO3?nH2O�������ط����������Ƴ���ͼ��ʾ����������ʾ��ͼ����

��t2��ʱ��ʣ�����Ϊ
������ʣ���4.0g������
��MgCO3?nH2O��ȫ�ֽ�Ļ�ѧ����ʽΪ��
| ||
| ||
��2�����ݶ�����̼��̼��þ�����������ϵ����
��3������ʵ��Ŀ�ķ�������������Һ������
��4������Cװ�õ����÷���Dװ�õ�����
��5������ʵ�鲽���������ʵ������ԭ��
��6�����ݲ�����������Ķ�����̼���� �ı仯������nֵ��Ӱ��
��7������ͼ���������ֽ���̼�����
��2��3��ʵ��Cװ��ƽ�����أ�1.1+1.11+1.09��g/3=1.1g
MgCO3?nH2O��CO2
84+18n 44
3.45g 1.1g
| 84+18n |
| 3.45g |
| 44 |
| 1.1g |
��ã�n=3
��3��������������֪Aװ����ʢ��������������Һ�������տ����еĶ�����̼����ֹ�����ɶ�����̼�����ĸ��ţ���
��4��Dװ�ü�ʯ�ҵ������Ƿ�ֹ�����е�CO2����Cװ�ã�������
��5��ʵ����������ֵ��ƫ�ԭ�������Bװ�����ɵ�CO2������ˮ����һͬ����Cװ��
��6���ٷ�Ӧ������û��ͨ�����������Ķ�����̼û��ȫ���ŵ�Cװ�ã�ʹ������̼������ƫС��������ƫ��
�ڳ���Cװ��ǰ��û��ͨ������ʹ�����ж�����̼��Cװ�����գ�ʹ������̼������ƫ��������ƫС��
��û��Aװ�ã�ʹ�����ж�����̼��Cװ�����գ�ʹ������̼������ƫ��������ƫС��
��û��Dװ�ã�ʹ�������ж�����̼����Cװ�����գ�ʹ������̼������ƫ��������ƫС��
��ϡ����μ�����̫�죬�����Ķ�����̼û��ȫ����Cװ�����գ�ʹ������̼������ƫС��������ƫ��
��Cװ����NaOH��ҺŨ�ȹ�����Ӱ�������̼���������Խ��û��Ӱ�죮
��7�����ɵڣ�2���ʼ����֪�þ���Ļ�ѧʽΪMgCO3?3H2O����ͼ���֪�þ���ֽ�����ȷֽ�����ˮ�����ᾧˮȫ��ʧȥ��̼��þ�ֽ���������þ��ˮ����t2��ʱ��ʣ����廯ѧʽΪMgCO3?xH2O�����ʱ�ֽ�Ļ�ѧ����ʽΪ
MgCO3?3H2O
| ||
138 84+x��18
13.8g 10.2g
| 138 |
| 13.8g |
| 84+18x |
| 10.2g |
x=1
�ʴ�ʱ���ʵĻ�ѧʽΪMgCO3?H2O
�ڸ��ݷֽ���̿�֪���շֽ������̼��þ��ȫ�ֽ���������þ��ˮ��������չ���������þ����ѧʽΪMgO
��MgCO3?nH2O��ȫ�ֽ�Ļ�ѧ����ʽΪMgCO3?3H2O
| ||
�ʴ�Ϊ����1���ټ�������� �ݻ����������
��2��3��ʵ��Cװ��ƽ�����أ�1.1+1.11+1.09��g/3=1.1g
MgCO3?nH2O��CO2
84+18n 44
3.45g 1.1g
| 84+18n |
| 3.45g |
| 44 |
| 1.1g |
��ã�n=3
��3��NaOH��Ũ����Һ
��4����ֹ�����е�CO2����Cװ�ã�������
��5��Bװ�����ɵ�CO2������ˮ����һͬ����Cװ��
��6���٢ݢڢۢ�
��7����MgCO3?H2O ��MgO ��MgCO3?3H2O
| ||

̼��þ���루MgCO3?nH2O��n=1��5���������㷺Ӧ����ұ���ͻ���ϼ�������Ʒ������Ϊ�ⶨ̼��þ������n��ֵ��ѧϰС�����������װ�ò�������3��ʵ�飺��������ϡ����ӷ���

���������ϡ���
a��̼��þ�������ȷֽ⣬����3�������
b����Է���������[Mr��MgCO3����84��Mr��H2O����18��Mr��MgO����40��Mr��CO2����44]
��ʵ�鲽�衿
��______��
��ȷ��ȡ3.45g MgCO3?nH2O����Bװ���У�����������
�۴��ɼ�a������һ��ʱ�����������Cװ�õ�������
�ܹرյ��ɼ�a����Һ©����������������ϡ���������ٲ�������Ϊֹ��
�ݴ��ɼ�a��______��
| �ࡡ�� | 1 | 2 | 3 |
| ʵ��ǰ | 228.00 | 228.00 | 228.00 |
| ʵ��� | 229.10 | 229.11 | 229.09 |
���ظ��������裻
��������ݽ��м��㣮
��ʵ����������ݴ�����
��1��Aװ����ʢ�ŵ���Һ��______����Ŀ����______
��2��Dװ�õ�������______��
��3������̼��þ�����е�nֵ����Ҫ�м�����̣�
��ʵ�鷴˼��
��4��ʵ����������ֵ��ƫ�������B��C����һ��______װ���Լ�����
��5������ѡ���У������ʵ����ƫ�����______��ƫС����______��
�ٷ�Ӧ������û��ͨ���������������� �ڳ���Cװ��ǰ��û��ͨ������
��û��Aװ�ã���������������������������û��Dװ�ã�
��ϡ����μ�����̫�죻�������������� ��Cװ����NaOH��ҺŨ�ȹ���
��6��Ϊ��ȷ�ⶨn��ֵ������ʦָ���£�С��ͬѧ��ȡ13.8g MgCO3?nH2O�������ط����������Ƴ�ͼE��ʾ����������ʾ��ͼ����
��t2��ʱ��ʣ�����Ϊ______���ѧʽ����
������ʣ���4.0g������______���ѧʽ����
��MgCO3?nH2O��ȫ�ֽ�Ļ�ѧ����ʽΪ��______��
̼��þ���루MgCO3·nH2O��n = 1~5���������㷺Ӧ����ұ���ͻ���ϼ�������Ʒ������Ϊ�ⶨ̼��þ������n��ֵ��ѧϰС�����������װ�ò�������3��ʵ�飺��������ϡ����ӷ���
���������ϡ���
a��̼��þ�������ȷֽ⣬����3�������
b����Է�����������Mr(MgCO3)��84��Mr(H2O)��18��Mr(MgO)��40��Mr(CO2)��44��
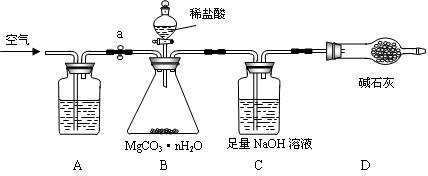
��ʵ�鲽�衿
�� �� ��
��ȷ��ȡ3.45g MgCO3·nH2O����Bװ���У�����������
�۴��ɼ�a������һ��ʱ�����������Cװ�õ�������
�ܹرյ��ɼ�a����Һ©����������������ϡ���������ٲ�������Ϊֹ��
�ݴ��ɼ�a�� �� ��
| �� �� | 1 | 2 | 3 |
| ʵ��ǰ | 228.00 | 228.00 | 228.00 |
| ʵ��� | 229.10 | 229.11 | 229.09 |
��ȷ����Cװ�õ����������ұ�����
���ظ��������裻
��������ݽ��м��㡣
��ʵ����������ݴ�����
��1������ʵ�鲽�裺�� �� ���� �� ��
��2�������̼��þ�����е�nֵ����Ҫ���м�����̣�3�֣�
��3��Aװ����ʢ�ŵ���Һ�� �� ��
��4��Dװ�õ������� �� ��
��ʵ�鷴˼��
��5��ʵ����������ֵ��ƫ�ԭ������� �� ��
��6������ѡ���У������ʵ����ƫ����� �� ��ƫС���� �� ��
�ٷ�Ӧ������û��ͨ������ �ڳ���Cװ��ǰ��û��ͨ������
��û��Aװ�ã� ��û��Dװ�ã�
��ϡ����μ�����̫�죻 ��Cװ����NaOH��ҺŨ�ȹ���
��7��Ϊ��ȷ�ⶨn��ֵ������ʦָ���£�С��ͬѧ��ȡ13.8 g MgCO3·nH2O�������ط����������Ƴ���ͼ��ʾ����������ʾ��ͼ����
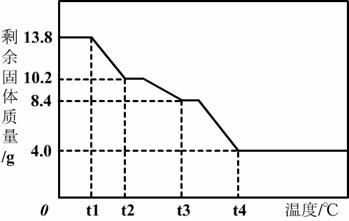
��t2��ʱ��ʣ�����Ϊ �� ������ѧʽ����
������ʣ���4.0 g������ �� ������ѧʽ����
��MgCO3·nH2O��ȫ�ֽ�Ļ�ѧ����ʽΪ�� �� ��
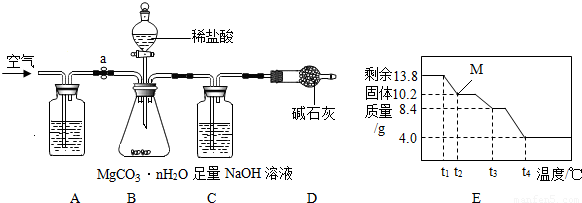
���������ϡ���
a��̼��þ�������ȷֽ⣬����3�������
b����Է���������[Mr��MgCO3����84��Mr��H2O����18��Mr��MgO����40��Mr��CO2����44]
��ʵ�鲽�衿
��______��
��ȷ��ȡ3.45g MgCO3?nH2O����Bװ���У�����������
�۴��ɼ�a������һ��ʱ�����������Cװ�õ�������
�ܹرյ��ɼ�a����Һ©����������������ϡ���������ٲ�������Ϊֹ��
�ݴ��ɼ�a��______��
| �� �� | 1 | 2 | 3 |
| ʵ��ǰ | 228.00 | 228.00 | 228.00 |
| ʵ��� | 229.10 | 229.11 | 229.09 |
���ظ��������裻
��������ݽ��м��㣮
��ʵ����������ݴ�����
��1��Aװ����ʢ�ŵ���Һ��______����Ŀ����______
��2��Dװ�õ�������______��
��3������̼��þ�����е�nֵ����Ҫ�м�����̣�
��ʵ�鷴˼��
��4��ʵ����������ֵ��ƫ�������B��C����һ��______װ���Լ�����
��5������ѡ���У������ʵ����ƫ�����______��ƫС����______��
�ٷ�Ӧ������û��ͨ������ �ڳ���Cװ��ǰ��û��ͨ������
��û��Aװ�ã� ��û��Dװ�ã�
��ϡ����μ�����̫�죻 ��Cװ����NaOH��ҺŨ�ȹ���
��6��Ϊ��ȷ�ⶨn��ֵ������ʦָ���£�С��ͬѧ��ȡ13.8g MgCO3?nH2O�������ط����������Ƴ�ͼE��ʾ����������ʾ��ͼ����
��t2��ʱ��ʣ�����Ϊ______���ѧʽ����
������ʣ���4.0g������______���ѧʽ����
��MgCO3?nH2O��ȫ�ֽ�Ļ�ѧ����ʽΪ��______ MgO+H2O+CO2��