��Ŀ����
��1��ij���������������������Ļ�ѧʽΪC4H2FeO4��ÿ��������������0.2g��������������ÿ��1��2���ٸ�����������C��H��Feԭ�Ӹ�����Ϊ______��
����ÿ�η���1���ò�������������Ԫ�ص�����Ϊ______mg��������ȡ������
��2��ѧУ����С��Ϊ�˲ⶨij������������Ʒ������������������ʵ�����г�ȡ������Ʒ5.8g�����ձ��У��ձ�������Ϊ30g����Ȼ���ټ���50gϡ���ᣬʹ֮ǡ����ȫ��Ӧ�������ձ����ձ������ʵ�������Ϊ85.6g����֪�÷�Ӧ�����ʲ����뷴Ӧ��������㣺
�ٲ���������������______
�ڸ�������Ʒ���������������Ƕ��٣����������һλС����
������ϡ������������������Ƕ��٣�
���𰸡���������1�����ݻ�ѧʽ����ʾ������ȷ��ԭ�Ӹ����ȣ��Լ���Ԫ�ص�������
��2����Ϊ����ϡ���ᷴӦ��������������������غ㶨�ɿ�֪����Ӧ����ٵ���������Ϊ����������������Ȼ���������ϡ���ᷴӦ�Ļ�ѧ����ʽ����������������������μӷ�Ӧ��������������������������������������������ϡ���������������������
����⣺��1�����ɸ��������������Ļ�ѧʽΪC4H2FeO4����֪������������C��H��Feԭ�Ӹ�����Ϊ��4��2��1��
�ڸ��������к�ijԪ�ص�����=�����ʵ�����×ijԪ�ص������������ɵã�ÿ�η���1���ò�������������Ԫ�ص�����Ϊ��0.2g×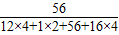 ×100%=0.066g=66mg��
×100%=0.066g=66mg��
��2����Ӧ��������������Ϊ��5.8g+30g+50g-85.6g=0.2g
��μӷ�Ӧ����������Ϊx����Ҫ����������Ϊy
Fe+H2SO4�TFeSO4+H2��
56 98 2
x y 0.2g

x=5.6g y=9.8g
����������������������Ϊ�� ×100%=96.6%
×100%=96.6%
����ϡ��������ʵ���������Ϊ�� ×100%=19.6%
×100%=19.6%
�𣺢ٲ�������������Ϊ0.2g��
�ڸ�������Ʒ��������������Ϊ96.6%��
������ϡ�������������������19.6%��
���������⿼��ѧ���Ի�ѧʽ��ԭ�Ӹ����ȣ���Ԫ�ص�������֪ʶ�����������գ������û�ѧ����ʽ�ļ��㣬�������÷�Ӧǰ���������IJ�ֵ�����������������Ǵ����ⳣ�õķ��������������������������������������˼�����Ѷȣ�
��2����Ϊ����ϡ���ᷴӦ��������������������غ㶨�ɿ�֪����Ӧ����ٵ���������Ϊ����������������Ȼ���������ϡ���ᷴӦ�Ļ�ѧ����ʽ����������������������μӷ�Ӧ��������������������������������������������ϡ���������������������
����⣺��1�����ɸ��������������Ļ�ѧʽΪC4H2FeO4����֪������������C��H��Feԭ�Ӹ�����Ϊ��4��2��1��
�ڸ��������к�ijԪ�ص�����=�����ʵ�����×ijԪ�ص������������ɵã�ÿ�η���1���ò�������������Ԫ�ص�����Ϊ��0.2g×
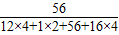 ×100%=0.066g=66mg��
×100%=0.066g=66mg����2����Ӧ��������������Ϊ��5.8g+30g+50g-85.6g=0.2g
��μӷ�Ӧ����������Ϊx����Ҫ����������Ϊy
Fe+H2SO4�TFeSO4+H2��
56 98 2
x y 0.2g

x=5.6g y=9.8g
������������������������
 ×100%=96.6%
×100%=96.6%����ϡ��������ʵ���������Ϊ��
 ×100%=19.6%
×100%=19.6%�𣺢ٲ�������������Ϊ0.2g��
�ڸ�������Ʒ��������������Ϊ96.6%��
������ϡ�������������������19.6%��
���������⿼��ѧ���Ի�ѧʽ��ԭ�Ӹ����ȣ���Ԫ�ص�������֪ʶ�����������գ������û�ѧ����ʽ�ļ��㣬�������÷�Ӧǰ���������IJ�ֵ�����������������Ǵ����ⳣ�õķ��������������������������������������˼�����Ѷȣ�

��ϰ��ϵ�д�
�����Ŀ
 ��ͼ��ijƷ�Ʋ����Լ��ı�ǩ����ش�
��ͼ��ijƷ�Ʋ����Լ��ı�ǩ����ش�