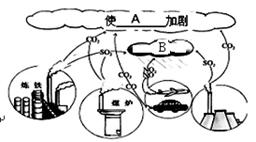
��Ŀ����
ѡ��̼��������ٶ�����̼�ŷţ���ÿλ����Ӧ�������κ�����
������������10�����ϴ����Լ���Լ0.02molCO2�ŷš�CO2��Ħ�������� ��6�� �������� ��7�� ���������ԡ����ԡ����� 0.02molCO2Լ���� ��8�� ��CO2���ӡ�
�ڡ���̼��ͨ������ʹ������Դ����������������Դ��д������ȼ�յĻ�ѧ����ʽ
��9�� ��
�ۻ��տ����õ���Դ������ֻ��еĽ������ֻ���о�к���ͭ�������ȡ�д�����Ļ�ѧʽ ��10�� ����ͼ��ʵ������ȡ����ͭ������ͼ��
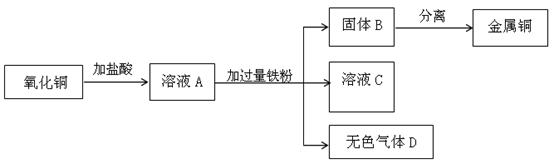
������ͭ�����ᷴӦ�Ļ�ѧ����ʽ�� ��11�� ��
����ҺA����������� ��12�� ��
���ڹ���B�м�������� ��13�� ��Һ����ͨ�� ��14�� �ķ��뷽���õ�����ͭ��
������������10�����ϴ����Լ���Լ0.02molCO2�ŷš�CO2��Ħ�������� ��6�� �������� ��7�� ���������ԡ����ԡ����� 0.02molCO2Լ���� ��8�� ��CO2���ӡ�
�ڡ���̼��ͨ������ʹ������Դ����������������Դ��д������ȼ�յĻ�ѧ����ʽ
��9�� ��
�ۻ��տ����õ���Դ������ֻ��еĽ������ֻ���о�к���ͭ�������ȡ�д�����Ļ�ѧʽ ��10�� ����ͼ��ʵ������ȡ����ͭ������ͼ��

������ͭ�����ᷴӦ�Ļ�ѧ����ʽ�� ��11�� ��
����ҺA����������� ��12�� ��
���ڹ���B�м�������� ��13�� ��Һ����ͨ�� ��14�� �ķ��뷽���õ�����ͭ��
��6��44 g/mol ��7������ ��8��1.204��1022 ��9��2H2��O2 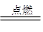 2H2O
2H2O
��10��Ag (11) CuO+2HCl==CuCl2+H2O (12) CuCl2 HCl
��13��HCl��CuCl2 (14) ����
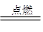 2H2O
2H2O��10��Ag (11) CuO+2HCl==CuCl2+H2O (12) CuCl2 HCl
��13��HCl��CuCl2 (14) ����
��CO2��Ħ��������44 g/mol�����������������0.02molCO2Լ����1.204��1022��CO2���ӣ�
������ȼ������ˮ����ѧ����ʽ��2H2��O2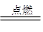 2H2O
2H2O
�����Ļ�ѧʽΪAg��
������ͭ�����ᷴӦ�����Ȼ�ͭ��ˮ����ѧ����ʽCuO+2HCl�TCuCl2+H2O��
�������������ҺA�����������CuCl2��HCl��
���������ᷴӦ�Լ������û���������ͭ���еĽ���ͭ���ڹ���B�м��������HCl��CuCl2��Һ����ͨ���Ĺ��˷��뷽���õ�����ͭ��
������ȼ������ˮ����ѧ����ʽ��2H2��O2
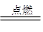 2H2O
2H2O�����Ļ�ѧʽΪAg��
������ͭ�����ᷴӦ�����Ȼ�ͭ��ˮ����ѧ����ʽCuO+2HCl�TCuCl2+H2O��
�������������ҺA�����������CuCl2��HCl��
���������ᷴӦ�Լ������û���������ͭ���еĽ���ͭ���ڹ���B�м��������HCl��CuCl2��Һ����ͨ���Ĺ��˷��뷽���õ�����ͭ��

��ϰ��ϵ�д�
�����Ŀ