��Ŀ����
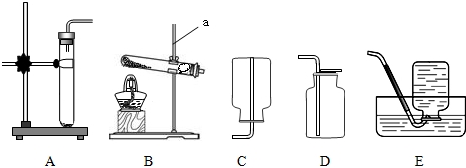
ʵ������ȡ���������װ����ͼ��ʾ����ش��������⣺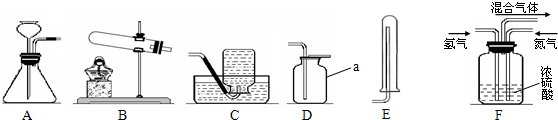
��1��д��װ��ͼ�б�����������ƣ�a
��2��ʵ������ȡ������̼�Ļ�ѧ����ʽΪ
��3��ʵ������װ��A��ȡ����������Cװ���ռ����������۲쵽���ܿ�������
��4��ѡ�������ռ�����ʱ��������������ʣ�����ɫ���ܶȢ��ܽ��Ԣܿ�ȼ�ԣ����б��뿼�ǵ���
��5��Ϊ�õ���������Ķ�����̼���壬����װ�ã���ͼ���ĵ��ܰ�������������˳���ǣ�ѡ����ĸ����
A��a��b��c��d B��b��a��c��d C��c��d��a��b D��d��c��b��a
��6��������һ����ɫ���д̼�����ζ����������ˮ�����壬�������Ƶ��ʡ����ᡢҩ���Ⱦ�ϵȣ�
��ʵ���ҳ��ü����Ȼ�狀���ʯ�ҵĹ�������ķ�����ȡ��������ʵ������ȡ���ռ�����Ӧѡ���װ�������
A��ˮ B�õ��ܲ���Ũ���� C�õ��ܲ���ϡ������ D�õ���©������ϡ������
�ڹ�ҵ���õ����������ϳɰ�����N2+3H2
2NH3��ʵ����ģ��ϳɰ������������£�
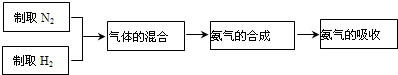
�����С�����Ļ�ϡ�����Fװ���н��еģ�Fװ�õ�������������һ�ǽ������������������ʹ������������ֻ�ϣ�����

��1��д��װ��ͼ�б�����������ƣ�a
����ƿ
����ƿ
����2��ʵ������ȡ������̼�Ļ�ѧ����ʽΪ
CaCO3+2HCl�TCaCl2+H2O+CO2��
CaCO3+2HCl�TCaCl2+H2O+CO2��
����ѡ�õķ������ռ����װ��ΪAD
AD
������ĸ������3��ʵ������װ��A��ȡ����������Cװ���ռ����������۲쵽���ܿ�������
����������
����������
�طų�ʱ�����ɽ����ռ�����4��ѡ�������ռ�����ʱ��������������ʣ�����ɫ���ܶȢ��ܽ��Ԣܿ�ȼ�ԣ����б��뿼�ǵ���
�ڢ�
�ڢ�
������ţ�����5��Ϊ�õ���������Ķ�����̼���壬����װ�ã���ͼ���ĵ��ܰ�������������˳���ǣ�ѡ����ĸ����
A��a��b��c��d B��b��a��c��d C��c��d��a��b D��d��c��b��a

��6��������һ����ɫ���д̼�����ζ����������ˮ�����壬�������Ƶ��ʡ����ᡢҩ���Ⱦ�ϵȣ�
��ʵ���ҳ��ü����Ȼ�狀���ʯ�ҵĹ�������ķ�����ȡ��������ʵ������ȡ���ռ�����Ӧѡ���װ�������
BE
BE
�������ķ���ʽ��2NH4Cl+Ca��OH��2
CaCl2+2H2O+2NH3��
| ||
2NH4Cl+Ca��OH��2
CaCl2+2H2O+2NH3��
�������Ϳ����������ﵽ��ը����V%����16%��25%����
| ||
����
����
���߾��緢����ը�����ж�����������֯����ˮ���ɰ�ˮ�������ܽ���֯�����ʣ�����β�������D
D
���գ�A��ˮ B�õ��ܲ���Ũ���� C�õ��ܲ���ϡ������ D�õ���©������ϡ������
�ڹ�ҵ���õ����������ϳɰ�����N2+3H2
| ||
| ���¸�ѹ |

�����С�����Ļ�ϡ�����Fװ���н��еģ�Fװ�õ�������������һ�ǽ������������������ʹ������������ֻ�ϣ�����
ʹ�����͵��������ȶ�
ʹ�����͵��������ȶ�
���Ӷ���ߵ����������������ʣ���������1�����ݳ����Ļ�ѧ���������ƽ��з������
��2��ʵ�����ô���ʯ��ϡ���ᷴӦ��ȡ������̼���ݷ�Ӧԭ����д����ʽ�����ݷ�Ӧ��״̬�ͷ�Ӧ����ѡ����װ�ã��ݶ�����̼���ܶȺ��ܽ���ѡ���ռ�װ�ã�
��3������ˮ���ռ�����ʱ��Ҫ�����ܿ���������������ð�����ռ�����ֹ�ռ�������������
��4��ѡ�������ռ�����ʱ��ͨ������������ܶȺ��ܽ��ԣ�
��5��Ҫ�õ��������������Ӧ���ȳ����ٸ�����ų����ţ���ϴ��װ�õ����ǡ������̳�����
��6���ٸ��ݷ������ռ�װ�õ�ѡȡ�������н�𣬲��ݷ�Ӧԭ����д����ʽ����ȼ������������������ը����ˮ�Լ��ԣ������ᷴӦ���ʿ�����������գ����ݻ��ϴ���������ܹ����ս϶��Һ�壻
��Fװ�õ�������������һ�ǽ������������������ʹ������������ֻ�ϣ�����ʹ�����͵��������ȶ���
��2��ʵ�����ô���ʯ��ϡ���ᷴӦ��ȡ������̼���ݷ�Ӧԭ����д����ʽ�����ݷ�Ӧ��״̬�ͷ�Ӧ����ѡ����װ�ã��ݶ�����̼���ܶȺ��ܽ���ѡ���ռ�װ�ã�
��3������ˮ���ռ�����ʱ��Ҫ�����ܿ���������������ð�����ռ�����ֹ�ռ�������������
��4��ѡ�������ռ�����ʱ��ͨ������������ܶȺ��ܽ��ԣ�
��5��Ҫ�õ��������������Ӧ���ȳ����ٸ�����ų����ţ���ϴ��װ�õ����ǡ������̳�����
��6���ٸ��ݷ������ռ�װ�õ�ѡȡ�������н�𣬲��ݷ�Ӧԭ����д����ʽ����ȼ������������������ը����ˮ�Լ��ԣ������ᷴӦ���ʿ�����������գ����ݻ��ϴ���������ܹ����ս϶��Һ�壻
��Fװ�õ�������������һ�ǽ������������������ʹ������������ֻ�ϣ�����ʹ�����͵��������ȶ���
����⣺��1����ż���ƿ��
��2��ʵ�����ô���ʯ��ϡ���ᷴӦ��ȡ������̼����Ӧ����ʽ��CaCO3+2HCl�TCaCl2+H2O+CO2�����÷�Ӧ������ȣ����ڹ�Һ�����ͣ���ѡ����װ��B��������̼���ܶȱȿ�������������ˮ�������������ſ������ռ���
��3������Cװ���ռ����������۲������ܿ����������������ȵطų�ʱ�����ɽ����ռ�����ʼ�����������л��п��������������ռ���
��4��ѡ�������ռ�����ʱ��ͨ������������ܶȺ��ܽ��ԣ�
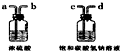
��5����ȡCO2�����г����е������Ǵ������лӷ��������Ȼ��������ˮ������CO2�����г�����HCl��ˮ������Ҫ�ȳ�ȥ�Ȼ������壬�ٸ��ͨ������װ��ʱӦ���ܽ��̹ܳ������Ӧ����c��d��a��b����ѡC��
��6�����ü����Ȼ�狀���ʯ�ҵĹ�������ķ�����ȡ����������ʽ��2NH4Cl+Ca��OH��2
CaCl2+2H2O+2NH3�����ɷ�Ӧԭ����֪�ǹ���ļ��ȷ�Ӧ��������װ��B������װ�ã��������ܶ�С�ڿ������ܶȣ���������ˮ�������ռ�װ����E����ȼ������������������ը����ˮ�Լ��ԣ������ᷴӦ���ʿ�����������գ����ݻ��ϴ���������ܹ����ս϶��Һ�壻
��Fװ�õ�������������һ�ǽ������������������ʹ������������ֻ�ϣ�����ͨ���۲�F�е����ݿ��ư�����������������ʹ�����͵��������ȶ����ﵽ��ѱ�����
�ʴ�Ϊ����1����ƿ��
��2��CaCO3+2HCl�TCaCl2+H2O+CO2���� AD��
��3�����������ȣ�
��4���ڢۣ�
��5��C��
��6����BE��2NH4Cl+Ca��OH��2
CaCl2+2H2O+2NH3��������D��
��ʹ�����͵��������ȶ���
��2��ʵ�����ô���ʯ��ϡ���ᷴӦ��ȡ������̼����Ӧ����ʽ��CaCO3+2HCl�TCaCl2+H2O+CO2�����÷�Ӧ������ȣ����ڹ�Һ�����ͣ���ѡ����װ��B��������̼���ܶȱȿ�������������ˮ�������������ſ������ռ���
��3������Cװ���ռ����������۲������ܿ����������������ȵطų�ʱ�����ɽ����ռ�����ʼ�����������л��п��������������ռ���
��4��ѡ�������ռ�����ʱ��ͨ������������ܶȺ��ܽ��ԣ�
��5����ȡCO2�����г����е������Ǵ������лӷ��������Ȼ��������ˮ������CO2�����г�����HCl��ˮ������Ҫ�ȳ�ȥ�Ȼ������壬�ٸ��ͨ������װ��ʱӦ���ܽ��̹ܳ������Ӧ����c��d��a��b����ѡC��
��6�����ü����Ȼ�狀���ʯ�ҵĹ�������ķ�����ȡ����������ʽ��2NH4Cl+Ca��OH��2
| ||
��Fװ�õ�������������һ�ǽ������������������ʹ������������ֻ�ϣ�����ͨ���۲�F�е����ݿ��ư�����������������ʹ�����͵��������ȶ����ﵽ��ѱ�����
�ʴ�Ϊ����1����ƿ��
��2��CaCO3+2HCl�TCaCl2+H2O+CO2���� AD��
��3�����������ȣ�
��4���ڢۣ�
��5��C��
��6����BE��2NH4Cl+Ca��OH��2
| ||
��ʹ�����͵��������ȶ���
�������������п�����Ҫ����֮һ����������ʵ��������ȡ����ķ�Ӧԭ��������װ�ú��ռ�װ�õ�ѡ����������ȷ�����Ĺؼ������������ճ�������������;�����ƣ�Ҫ�õ��������������Ӧ���ȳ����ٸ�����ų����ţ�ϴ��װ�õ����ǡ������̳������ܽϺÿ���ѧ��Ӧ��֪ʶ��������

��ϰ��ϵ�д�
�����Ŀ