��Ŀ����
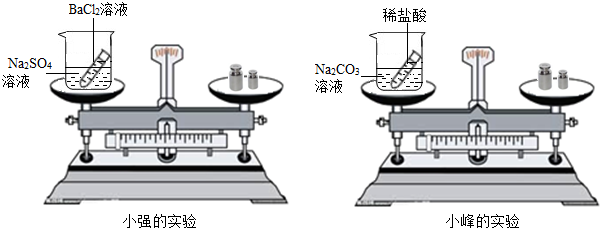
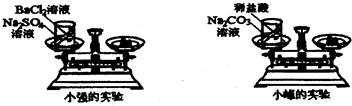
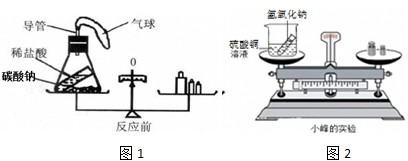
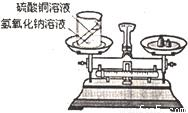
��ѧϰ�������غ㶨�ɺ�С����С������ʵ���ң���������ͼ��ʾ��ʵ��װ�ã���֤������������Һ������ͭ��Һ��Ӧ�Ƿ��������غ㶨�ɵģ���1����д���÷�Ӧ�Ļ�ѧ���� ��
��2��ʵ�����������������ʱС�����֣�ԭ������������Һ�Ƿ��ڳ��������У��������ǶԸո���ɵ�ʵ����������ʣ�
[�������]�����������Ƿ���ʣ����������������Һ�������ʣ������ʡ�����������������ͭ��Һ��ӦΪʲô��Ȼ���������غ㶨�ɣ�
[��������]����ͭ�ε��ܽ��Ա���20�棩
| ������\������ | SO42- | N03- | PO43- | Cl- | C032- |
| Cu2+ | �� | �� | �� | �� | �� |
[���ʵ��]����ȷ�����������Ƿ���ʣ�
| ʵ����� | ʵ������ | ���� |
��
�ڡ����ʡ�������������Һ������ͭ��Һ��Ӧ��Ϊʲô��Ȼ���������غ㶨�ɣ�
��
���𰸡���������1�����ݸ��ֽ��ԭ������ѧ����ʽ����д������н��
��3��[���ʵ��]����������һ�ּ���ڷ��ã��������տ����е�������̼������̼���ƣ����Կ��������������м��ᣬͨ���Ƿ������������������֤���Ƿ���ʣ�
[���������]�ɸ��������غ㶨�ɽ��з������ص������Ӧ����������Ƿ��������ձ��У�
����⣺��1������������Һ������ͭ��Һ��Ϸ������ֽⷴӦ����������ļ���Σ�2NaOH+CuSO4�TNa2SO4+Cu��OH��2����3��[���ʵ��]������������һ�ּ���ڷ��ã��������տ����е�������̼������̼���ƣ����Կ��������������еμ���������ᣬͨ���Ƿ������������������֤���Ƿ���ʣ��������ݲ�����֤���Ѿ����ʣ�
[���������]���������Ʊ���������̼���ƣ�����ʵ����������еμ�����ͭ��Һ�������ܷ���̼���ƺ�����ͭ��Ӧ
Na2CO3+CuSO4=Na2SO4+CuCO3��
�ڡ����ʡ�������������Һ������ͭ��Һ��Ӧ����Ӧǰ��Ԫ�ص����ࡢ�������䣻ͬʱ��Ӧ������û�������������Ӧ�������������ձ��У����Է�Ӧǰ������ձ���������ı䣬��Ȼ���������غ㶨�ɣ�
������1��2NaOH+CuSO4�TNa2SO4+Cu��OH��2��
��3��[���ʵ��]
[���������]
��Na2CO3+CuSO4=Na2SO4+CuCO3��
�ڡ����ʡ�������������Һ������ͭ��Һ��Ӧ����Ӧǰ��Ԫ�ص����ࡢ�������䣻̼������Һ������ͭ��Һ��Ӧ����Ӧ������û�����������̼������Һ������ͭ��Һ��Ӧ����Ӧ�������������ձ��У�
������������һ���ۺ�ʵ���⣬ͨ���������Ƶı��ʵ�̽�֣�������ѧ���Ի�ѧʵ�鷽������ƺ����ۣ���ʵ�����ķ������жϵ�������
��3��[���ʵ��]����������һ�ּ���ڷ��ã��������տ����е�������̼������̼���ƣ����Կ��������������м��ᣬͨ���Ƿ������������������֤���Ƿ���ʣ�
[���������]�ɸ��������غ㶨�ɽ��з������ص������Ӧ����������Ƿ��������ձ��У�
����⣺��1������������Һ������ͭ��Һ��Ϸ������ֽⷴӦ����������ļ���Σ�2NaOH+CuSO4�TNa2SO4+Cu��OH��2����3��[���ʵ��]������������һ�ּ���ڷ��ã��������տ����е�������̼������̼���ƣ����Կ��������������еμ���������ᣬͨ���Ƿ������������������֤���Ƿ���ʣ��������ݲ�����֤���Ѿ����ʣ�
[���������]���������Ʊ���������̼���ƣ�����ʵ����������еμ�����ͭ��Һ�������ܷ���̼���ƺ�����ͭ��Ӧ
Na2CO3+CuSO4=Na2SO4+CuCO3��
�ڡ����ʡ�������������Һ������ͭ��Һ��Ӧ����Ӧǰ��Ԫ�ص����ࡢ�������䣻ͬʱ��Ӧ������û�������������Ӧ�������������ձ��У����Է�Ӧǰ������ձ���������ı䣬��Ȼ���������غ㶨�ɣ�
������1��2NaOH+CuSO4�TNa2SO4+Cu��OH��2��
��3��[���ʵ��]
| ʵ����� | ʵ������ | ���� |
| ������������Һ�еμ����ᣨ��ϡ���ᣩ | �����ݲ��� | ���� |
��Na2CO3+CuSO4=Na2SO4+CuCO3��
�ڡ����ʡ�������������Һ������ͭ��Һ��Ӧ����Ӧǰ��Ԫ�ص����ࡢ�������䣻̼������Һ������ͭ��Һ��Ӧ����Ӧ������û�����������̼������Һ������ͭ��Һ��Ӧ����Ӧ�������������ձ��У�
������������һ���ۺ�ʵ���⣬ͨ���������Ƶı��ʵ�̽�֣�������ѧ���Ի�ѧʵ�鷽������ƺ����ۣ���ʵ�����ķ������жϵ�������

��ϰ��ϵ�д�
�����Ŀ
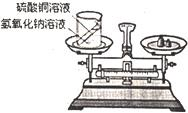 24����ѧϰ�������غ㶨�ɺ�С����С������ʵ���ң���������ͼ��ʾ��ʵ��װ�ã���֤������������Һ������ͭ��Һ��Ӧ�Ƿ��������غ㶨�ɵģ�
24����ѧϰ�������غ㶨�ɺ�С����С������ʵ���ң���������ͼ��ʾ��ʵ��װ�ã���֤������������Һ������ͭ��Һ��Ӧ�Ƿ��������غ㶨�ɵģ�