��Ŀ����
 4��17�գ�������������ʯ���µ�һ���������ֺ�ij��ѧ��ѧ��ȤС����̽�������ֳ������Ŀ������Ƿ���CO���壮���ʵ�鷽�����£�
4��17�գ�������������ʯ���µ�һ���������ֺ�ij��ѧ��ѧ��ȤС����̽�������ֳ������Ŀ������Ƿ���CO���壮���ʵ�鷽�����£�[ʵ��Ŀ��]ȷ��������Ʒ���Ƿ���CO��
[ʵ��ԭ��]���ȳ�ȥ�ռ��Ŀ�����Ʒ�е�����������ɣ���
����Fe2O3��COת�������Լ�⣨�������Ʒ�в�����CO֮�����������Fe2O3��Ӧ�����ʣ���
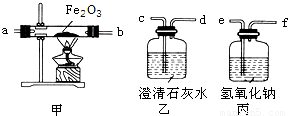
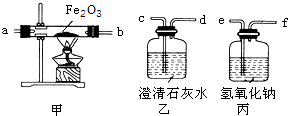
[ʵ��������װ��]��������ǵ���ƻش��������⣺
��1����ʵ��ʱ����������������˳���ǣ������ҡ��ס��ң���������Ʒ��������װ���еĽӿ�˳���ǣ�e��
fcdabcd
fcdabcd
��д������װ���з�����Ӧ�Ļ�ѧ����ʽ����3CO+Fe2O3
2Fe+3CO2
| ||
3CO+Fe2O3
2Fe+3CO2
����
| ||
2NaOH+CO2�TNa2CO3+H2O
2NaOH+CO2�TNa2CO3+H2O
��������װ����Ҫʹ�����Σ���һ�ε�����������ԭ������Ʒ�еĶ�����̼�Ƿ����
����ԭ������Ʒ�еĶ�����̼�Ƿ����
����2������ʵ���г��ֵ�
����ҩƷ�ɺ��ڣ����һ��ͨ����ʯ��ˮ�����ǣ��ڶ���ͨ����ʯ��ˮ����ǣ�
����ҩƷ�ɺ��ڣ����һ��ͨ����ʯ��ˮ�����ǣ��ڶ���ͨ����ʯ��ˮ����ǣ�
�������жϳ������ֳ������Ŀ����к���CO��������Ⱦ�������CO������������P�ܵ���������������NO2��SO2
NO2��SO2
���ȣ���3���ӻ��������ĽǶȿ��ǣ�����Ϊ��ʵ�������Ƿ����ȱ�ݣ�����У�����θģ�
�У���d����β����ȼ������d���������ռ�β����
�У���d����β����ȼ������d���������ռ�β����
������������������Һ�ܹ����ն�����̼���壬������̼��ʹ�����ʯ��ˮ����ǣ�һ����̼�ж����ܹ���Ⱦ������
����⣺��1����Ϊ�����еĶ�����̼��ʵ���и������ã�Ӧ�ó�ȥ��������������Һ���ն�����̼���ó����ʯ��ˮ���Լ�������еĶ�����̼�Ƿ�������ܹ���װ��һ����̼��ԭ��������ͨ������ʯ��ˮ��֤���������Ƿ��ж�����̼������ͨ��Һ����ӵ�װ��Ӧ���ܽ����̹ܳ������װ�õ�����˳��ӦΪefcdabcd�����з�Ӧ�Ļ�ѧ����ʽΪ3CO+Fe2O3
2Fe+3CO2 ���з�Ӧ�ķ���ʽΪ2NaOH+CO2�TNa2CO3+H2O����һ����װ�õ������Ǽ���ԭ������Ʒ�еĶ�����̼�Ƿ������
��2�������һ����̼����һ����̼�ܺ���������Ӧ���ɺ�ɫ�����ۺͶ�����̼��������̼��ʹ�����ʯ��ˮ����ǣ�Ŀǰ���������Ⱦ�����ĿΪ��һ����̼���������������������������ȣ����������Ͷ��������ǵ����������Ҫ���壮
��3��һ����̼�ж����ܹ���Ⱦ������Ҫ��β�����д����������ķ����ǣ���d����β����ȼ����d���������ռ�β���ʴ�Ϊ����1��fcdabcd 3CO+Fe2O3
2Fe+3CO2 2NaOH+CO2�TNa2CO3+H2O
����ԭ������Ʒ�еĶ�����̼�Ƿ����
��2������ҩƷ�ɺ��ڣ����һ��ͨ����ʯ��ˮ�����ǣ��ڶ���ͨ����ʯ��ˮ����ǣ� NO2��SO2
��3���У���d����β����ȼ������d���������ռ�β�����������֣�
| ||
��2�������һ����̼����һ����̼�ܺ���������Ӧ���ɺ�ɫ�����ۺͶ�����̼��������̼��ʹ�����ʯ��ˮ����ǣ�Ŀǰ���������Ⱦ�����ĿΪ��һ����̼���������������������������ȣ����������Ͷ��������ǵ����������Ҫ���壮
��3��һ����̼�ж����ܹ���Ⱦ������Ҫ��β�����д����������ķ����ǣ���d����β����ȼ����d���������ռ�β���ʴ�Ϊ����1��fcdabcd 3CO+Fe2O3
| ||
����ԭ������Ʒ�еĶ�����̼�Ƿ����
��2������ҩƷ�ɺ��ڣ����һ��ͨ����ʯ��ˮ�����ǣ��ڶ���ͨ����ʯ��ˮ����ǣ� NO2��SO2
��3���У���d����β����ȼ������d���������ռ�β�����������֣�
������������Ҫ����һ����̼�Ļ�ԭ�ԣ��Լ�ʵ�����������ֻ�г��������һ����̼�����ʲ��ܶ���ط��������������ȷ���жϣ�

��ϰ��ϵ�д�
 ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
�����Ŀ
 4��17�գ�������������ʯ���µ�һ���������ֺ�ij��ѧ��ѧ��ȤС����̽�������ֳ������Ŀ������Ƿ���CO���壮���ʵ�鷽�����£�
4��17�գ�������������ʯ���µ�һ���������ֺ�ij��ѧ��ѧ��ȤС����̽�������ֳ������Ŀ������Ƿ���CO���壮���ʵ�鷽�����£�