��Ŀ����
��ҵ�����г�����ʳ�Σ���Ҫ�ɷ�ΪNa2CO3����ΪNaCl����ij������Ϊ�˲ⶨһ��������Na2CO3���������������������·���ʵ�飺ȡ������Ʒ�����ձ��У���ˮ����ܽ⣬�����μ�ϡ���ᣬ������������Ϊֹ���й����������ʾ����1��������Ʒ��Na2CO3������������
��2���ձ�����Һ�����ʵ�����������
| ���� | ��Ʒ���� | ˮ������ | ����ϡ�������� | ��Ӧ����Һ���� |
| ������g�� | 22 | 50 | 56.8 | 120 |
���𰸡���������1������̼���ƺ�ϡ���ᷴӦ�����Ȼ��ơ�ˮ��������̼�����������غ㶨�ɿ�֪��Ӧǰ����������ľ������ɵĶ�����̼��Ȼ����ݻ�ѧ����ʽ���㣮
��2�����÷�Ӧ����Һ���Ȼ��Ƶ���Һ������Na2CO3+2HCl�T2NaCl+CO2��+H2O����Ȼ��Ƶ�����������������������=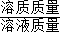 ×100%������
×100%������
����⣺���������غ㶨�ɵ�CO2=22+50+56.8-120=8.8g
�����ɵ�Na2CO3������ΪX�����ɵ�NaCl������ΪY����
Na2CO3+2HCl�T2NaCl+CO2��+H2O
106 117 44
X Y 8.8g
��1��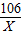 =
=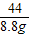
��֮��X=21.2g
Na2CO3����������=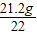 ×100%=96.4%
×100%=96.4%
��2�� =
=
��֮��Y=23.4g
NaCl����������=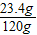 ×100%=19.5%
×100%=19.5%
���ձ�����Һ��������������Ϊ19.5%
������ֻҪ���巴Ӧǰ��������������Ϊ�����˶�����̼����ļ��ɸ��ݷ�Ӧ�Ļ�ѧ����ʽ�����⣬��Һ�ͻ�ѧ��Ӧ�ںϵ���Ŀ�����ۺ��Ե����ͣ�Ҫ��Ƚϸߣ���
��2�����÷�Ӧ����Һ���Ȼ��Ƶ���Һ������Na2CO3+2HCl�T2NaCl+CO2��+H2O����Ȼ��Ƶ�����������������������=
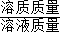 ×100%������
×100%����������⣺���������غ㶨�ɵ�CO2=22+50+56.8-120=8.8g
�����ɵ�Na2CO3������ΪX�����ɵ�NaCl������ΪY����
Na2CO3+2HCl�T2NaCl+CO2��+H2O
106 117 44
X Y 8.8g
��1��
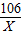 =
=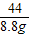
��֮��X=21.2g
Na2CO3����������=
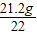 ×100%=96.4%
×100%=96.4%��2��
 =
=
��֮��Y=23.4g
NaCl����������=
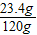 ×100%=19.5%
×100%=19.5%���ձ�����Һ��������������Ϊ19.5%
������ֻҪ���巴Ӧǰ��������������Ϊ�����˶�����̼����ļ��ɸ��ݷ�Ӧ�Ļ�ѧ����ʽ�����⣬��Һ�ͻ�ѧ��Ӧ�ںϵ���Ŀ�����ۺ��Ե����ͣ�Ҫ��Ƚϸߣ���

��ϰ��ϵ�д�
 Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д� ������������Ծ�ϵ�д�
������������Ծ�ϵ�д� �������Ӳ�ϵ�д�
�������Ӳ�ϵ�д�
�����Ŀ
��ҵ�����г�����ʳ�Σ���Ҫ�ɷ�ΪNa2CO3����ΪNaCl����ij������Ϊ�˲ⶨһ��������Na2CO3���������������������·���ʵ�飺ȡ������Ʒ�����ձ��У���ˮ����ܽ⣬�����μ�ϡ���ᣬ������������Ϊֹ���й��������±���ʾ������Ʒ��Na2CO3������������
| ���� | ��Ʒ���� | ˮ������ | ����ϡ�������� | ��Ӧ����Һ���� |
| ������g�� | 22 | 50 | 56.8 | 120 |
