��Ŀ����
����Ŀ��������茶��壨NH4Al(SO4)2��xH2O����һ����;�㷺�ĺ����������ȤС���ڿ�����Աָ���£����ʵ���о�������茶������ȷֽ�IJ��
���������ϣ���SO2����ϡKMnO4��Һ��Ӧʹ����ɫ��
��SO3+H2O=H2SO4��
����������ȷֽ�ɵõ�������������
�ܼ�ʯ����CaO��NaOH�Ļ���
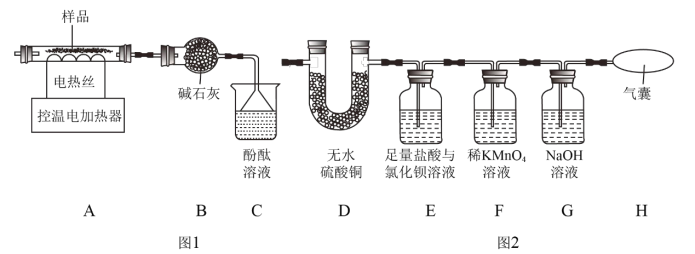
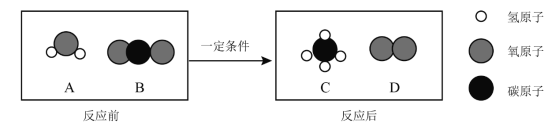
��1��������茶���ֽ����Ķ���̽��
��װ��C�й۲쵽��̪��Һ���ɫ���ɴ˿�֪�ֽ�IJ�������_____��д��ѧʽ����
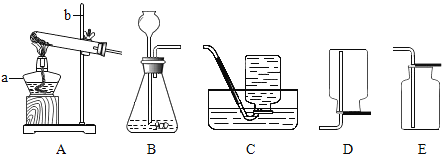
��ijͬѧ��Ϊ������茶������ȷֽ�IJ����л���H2O��SO2��SO3��N2��Ϊ������֤����ͼ1�е�װ��A��ͼ2��ʾװ�����ӽ���ʵ�顣ʵ���У�װ��D��������_____��֤����H2O���ɣ�װ��E��������_____��֤����SO3���ɣ���װ��F��������_____��H����û���ʹ�֤��û��SO2��N2���ɡ�
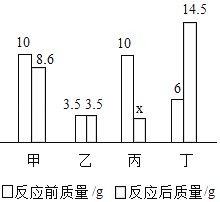
��2��������茶���ɷּ��ֽ����Ķ���̽��Ϊȷ��������茶������ɣ���ȡ45.3g��Ʒ�ڿ����г������ȣ��ⶨʣ������������¶ȱ仯��������ͼ��ʾ��
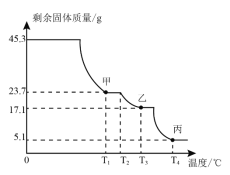
�ٹ������T1���պ���ȫʧȥ�ᾧˮ�IJ����x=_____��
��T3��ʱ�����ҵijɷ���_____������ĸ����
A��Al2(SO4)3
B��Al(OH)3
C��Al2(SO4)3��Al(OH)3
���𰸡�NH3 ��ɫ������� �а�ɫ�������� ϡ���������Һ����ɫ 12 A
��������
��1����װ��C�й۲쵽��̪��Һ���ɫ���ɴ˿�֪�ֽ�IJ�������NH3��
��ijͬѧ��Ϊ������茶������ȷֽ�IJ����л���H2O��SO2��SO3��N2��Ϊ������֤����ͼ1�е�װ��A��ͼ2��ʾװ�����ӽ���ʵ�顣ʵ���У�װ��D�������ǰ�ɫ���������֤����H2O���ɣ�װ��E���������а�ɫ�������ɣ�֤����SO3���ɣ���װ��F��������ϡ���������Һ����ɫ��H����û���ʹ�֤��û��SO2��N2���ɡ�
��2���ٹ������T1��պ���ȫʧȥ�ᾧˮ�IJ�������һ�μ��ٵ�����Ϊ��45.3g-23.7g=21.6g��ˮ��������Ϊ��![]() �����Խᾧˮ�ĸ���x=12��
�����Խᾧˮ�ĸ���x=12��
�����������еķ���������342��T3��ʱ�����ҵ�����������17.1g��������֪��������������ѧʽΪ��Al2��SO4��3��