��Ŀ����
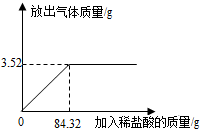 ��2013?������Ϊ�ⶨʯ��ʯ��Ʒ��̼��Ƶ�����������ijѧϰС�����������ʵ��̽����ȡ10gʯ��ʯ��Ʒ�����ձ��У�����ϡ���������ٲ�������Ϊֹ��ʯ��ʯ�е����ʲ�����ˮ��Ҳ����Ӧ�����������˼���ϡ�����������ų���������������ͼ����ͼ����
��2013?������Ϊ�ⶨʯ��ʯ��Ʒ��̼��Ƶ�����������ijѧϰС�����������ʵ��̽����ȡ10gʯ��ʯ��Ʒ�����ձ��У�����ϡ���������ٲ�������Ϊֹ��ʯ��ʯ�е����ʲ�����ˮ��Ҳ����Ӧ�����������˼���ϡ�����������ų���������������ͼ����ͼ������1��̼�����ȫ��Ӧ��ȥϡ���������Ϊ
84.32
84.32
g����2����ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ
80%
80%
����3���������ʯ��ʯǡ����ȫ��Ӧ��������Һ���������������Ƕ��٣����淶д��������̣�
��������1��ͼ���ת�۵��Ƕ���ǡ�÷�Ӧ�ĵ㣬�۲�ͼ���֪��ʱ��ȥϡ���������Ϊ84.32g��
��2�����ݶ�����̼�����������̼��Ƶ�������������
��100%�������ʯ��ʯ��Ʒ��̼��Ƶ�����������
��3�����ݶ�����̼��������������ɵ��Ȼ��Ƶ�������������Һ������=��Ӧǰ����ݵ�����֮��-���������-���ʵ��������������ʵ������������
��2�����ݶ�����̼�����������̼��Ƶ�������������
| ̼��Ƶ����� |
| ʯ��ʯ�������� |
��3�����ݶ�����̼��������������ɵ��Ȼ��Ƶ�������������Һ������=��Ӧǰ����ݵ�����֮��-���������-���ʵ��������������ʵ������������
����⣺��1��ͼ���ת�۵��Ƕ���ǡ�÷�Ӧ�ĵ㣬�۲�ͼ���֪��ʱ��ȥϡ���������Ϊ84.32g��
��2����ͼ���֪������Ʒ��������ᷴӦ���ɶ�����̼3.52g��
��̼��Ƶ�����Ϊx�����ɵ��Ȼ�������Ϊy
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 111 44
x y 3.52g
=
x=8g
=
y=8.88g
��ʯ��ʯ��Ʒ��̼��Ƶ���������=
��100%=80%
��3����Ӧ���Ȼ�����Һ������Ϊ��84.32g+10g-3.52g-��10g-8g��=88.8g
������Һ���������������ǣ�
��100%=10%
�ʴ�Ϊ����1��84.32��
��2��80%��
��3��������Һ�����ʵ���������Ϊ10%��
��2����ͼ���֪������Ʒ��������ᷴӦ���ɶ�����̼3.52g��
��̼��Ƶ�����Ϊx�����ɵ��Ȼ�������Ϊy
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 111 44
x y 3.52g
| 100 |
| x |
| 44 |
| 3.52g |
| 111 |
| y |
| 44 |
| 3.52g |
��ʯ��ʯ��Ʒ��̼��Ƶ���������=
| 8g |
| 10g |
��3����Ӧ���Ȼ�����Һ������Ϊ��84.32g+10g-3.52g-��10g-8g��=88.8g
������Һ���������������ǣ�
| 8.88g |
| 88.8g |
�ʴ�Ϊ����1��84.32��
��2��80%��
��3��������Һ�����ʵ���������Ϊ10%��
������������һ������ͼ�����Ŀ�����ʱע�����ͼ�����㡢ת�۵���յ㼰�仯���ƣ�������ת�۵�ͨ���ǻ�ѧ��Ӧǡ����ɵĵ㣻��Ӧ��������Һ�����������������dz��л�ѧ�����һ���ص���ѵ㣬���㷴Ӧ����Һ�������ķ���һ���ǣ���Ӧ����Һ������=��Ӧǰ����ݵ�����֮��-���������-�����������ʣ���������

��ϰ��ϵ�д�
 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�
�����Ŀ
 ��2013?���������۽Ƕ��ϣ����ѧ��Ӧ������ģ��ͼ������ı�ʾ�䷴Ӧ�����Ĺ��̣�ͼ�С�
��2013?���������۽Ƕ��ϣ����ѧ��Ӧ������ģ��ͼ������ı�ʾ�䷴Ӧ�����Ĺ��̣�ͼ�С� ���͡�
���͡� ���ֱ��ʾ��ͬԪ�ص�ԭ�ӣ����й���ͼ��ʾ��Ӧ��˵������ȷ���ǣ�������
���ֱ��ʾ��ͬԪ�ص�ԭ�ӣ����й���ͼ��ʾ��Ӧ��˵������ȷ���ǣ������� ��2013?�����������ز���524������Ӫ���ḻ���������ۡ������ʡ�����ά���ء�����п���Ƶȣ�
��2013?�����������ز���524������Ӫ���ḻ���������ۡ������ʡ�����ά���ء�����п���Ƶȣ�

