��Ŀ����
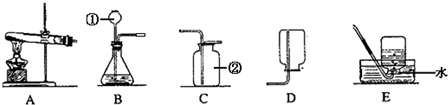
���������ʵ��װ��ͼ�ش��й����⣺
��1����д��ͼ�����������ƣ���
��2��ʵ�����ﳣ�ô���ʯ��ϡ���ᷴӦ��ȡ������̼����ѧ����ʽ��
��3������A��Ϊ�����ķ���װ�ã���ѧ����ʽ��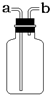
��4��ʵ�����ﳣ��п����ϡ���ᷴӦ��ȡ��������ѧ����ʽ��

��1����д��ͼ�����������ƣ���
����©��
����©��
��������ƿ
����ƿ
����2��ʵ�����ﳣ�ô���ʯ��ϡ���ᷴӦ��ȡ������̼����ѧ����ʽ��
CaCO3+2HCl�TCaCl2+H2O+CO2��
CaCO3+2HCl�TCaCl2+H2O+CO2��
����ѡ�õķ���װ�ú��ռ�װ����BC
BC
��ѡ����ĸ��������÷���װ�������Եķ�������ֹˮ�У���ˮ������©���¶���©�����ˮ��û��©���ڣ���ֹˮ�м�ס�����Ҷ˵��齺�ܣ��ټ�����©�����ˮ�����©����ˮλ����©���⣬��һ��ʱ��ˮλ���ֲ��䣬��˵�����������ã�
��ֹˮ�У���ˮ������©���¶���©�����ˮ��û��©���ڣ���ֹˮ�м�ס�����Ҷ˵��齺�ܣ��ټ�����©�����ˮ�����©����ˮλ����©���⣬��һ��ʱ��ˮλ���ֲ��䣬��˵�����������ã�
��ʵ���ҳ��ó���ʯ��ˮ���������̼����ѧ����ʽ��CO2+Ca��OH��2�TCaCO3��+H2O
CO2+Ca��OH��2�TCaCO3��+H2O
����3������A��Ϊ�����ķ���װ�ã���ѧ����ʽ��
2KMnO4
K2MnO4+MnO2+O2��
| ||
2KMnO4
K2MnO4+MnO2+O2��
��
| ||

��4��ʵ�����ﳣ��п����ϡ���ᷴӦ��ȡ��������ѧ����ʽ��
Zn+H2SO4�TZnSO4+H2��
Zn+H2SO4�TZnSO4+H2��
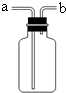
���ռ�������װ������ͼ��ʾ����װ���������ſ������ռ�����������Ӧ��b
b
�ˣ�ѡ�a����b������ͬ��ͨ�룻��������ˮ���ռ�����ʱ��ƿ����װ��ˮ������Ӧ��b
b
��ͨ�룮��������1�����ݳ�����������ʶ�����
��2�����ݷ�Ӧ���״̬������������������жϷ�Ӧװ�ü��ռ�װ�ã�����װ��B�������Եķ������ؼ�Ҫ�γ��ܱ���ϵ��ͬʱ�۲��ܱ���ϵ�Ƿ��ȶ���
��3��Aװ���ǹ��巴Ӧ��ȡ�����װ�ã���֮�Թ����������Դ�װ���Ǹ��������ȡ������װ�ã�
��4��ʵ������ȡ����һ��ѡ��п����ϡ������ȡ�������������ܶȼ�ʵ���Ҫ����������ռ����з����жϣ�
��2�����ݷ�Ӧ���״̬������������������жϷ�Ӧװ�ü��ռ�װ�ã�����װ��B�������Եķ������ؼ�Ҫ�γ��ܱ���ϵ��ͬʱ�۲��ܱ���ϵ�Ƿ��ȶ���
��3��Aװ���ǹ��巴Ӧ��ȡ�����װ�ã���֮�Թ����������Դ�װ���Ǹ��������ȡ������װ�ã�
��4��ʵ������ȡ����һ��ѡ��п����ϡ������ȡ�������������ܶȼ�ʵ���Ҫ����������ռ����з����жϣ�
����⣺��1����д������������ƣ��ٳ���©���ڼ���ƿ
��2��������̼����ȡһ��ѡ��̼��ƹ��弰ϡ����Һ�壬����ѡ����ȡװ����B��������̼���ܶȱȿ�������������ˮ���Բ��������ſ������ռ��Ϻ��ʣ�����װ��B�������Եķ������ؼ�Ҫ�γ��ܱ���ϵ��ͬʱ�۲��ܱ���ϵ�Ƿ��ȶ�������Ϊ����ֹˮ�У���ˮ������©���¶���Һ������ʱ������ֹˮ�У�������ˮ����Һ���Ƿ��ܹ�����������߹۲�Һ���Ƿ�仯��
��3��Aװ���ǹ��巴Ӧ��ȡ�����װ�ã���֮�Թ����������Դ�װ���Ǹ��������ȡ������װ�ã����Ⱥ�����ɶ������̡�����ؼ�������
��4��ʵ������ȡ����һ��ѡ��п����ϡ������ȡ�����������ſ������ռ�������ƿ��װ�������������ܶȱȿ���С��Ӧ�Ӷ̹ܽ����������ӳ����ų���
����������ˮ���ռ�����ʱ��ƿ����װ��ˮ������Ӧ�Ӷ̹ܽ�����ˮ�ӳ����ų���
�ʴ�Ϊ����1���ٳ���©�� �ڼ���ƿ
��2��CaCO3+2HCl�TCaCl2+H2O+CO2�� BC
��©�����ˮ��û��©���ڣ���ֹˮ�м�ס�����Ҷ˵��齺�ܣ��ټ�����©�����ˮ�����©����ˮλ����©���⣬��һ��ʱ��ˮλ���ֲ��䣬��˵�����������ã������������ɣ�
CO2+Ca��OH��2�TCaCO3��+H2O
��3��2KMnO4
K2MnO4+MnO2+O2��
��4��Zn+H2SO4�TZnSO4+H2�� b b
��2��������̼����ȡһ��ѡ��̼��ƹ��弰ϡ����Һ�壬����ѡ����ȡװ����B��������̼���ܶȱȿ�������������ˮ���Բ��������ſ������ռ��Ϻ��ʣ�����װ��B�������Եķ������ؼ�Ҫ�γ��ܱ���ϵ��ͬʱ�۲��ܱ���ϵ�Ƿ��ȶ�������Ϊ����ֹˮ�У���ˮ������©���¶���Һ������ʱ������ֹˮ�У�������ˮ����Һ���Ƿ��ܹ�����������߹۲�Һ���Ƿ�仯��
��3��Aװ���ǹ��巴Ӧ��ȡ�����װ�ã���֮�Թ����������Դ�װ���Ǹ��������ȡ������װ�ã����Ⱥ�����ɶ������̡�����ؼ�������
��4��ʵ������ȡ����һ��ѡ��п����ϡ������ȡ�����������ſ������ռ�������ƿ��װ�������������ܶȱȿ���С��Ӧ�Ӷ̹ܽ����������ӳ����ų���
����������ˮ���ռ�����ʱ��ƿ����װ��ˮ������Ӧ�Ӷ̹ܽ�����ˮ�ӳ����ų���
�ʴ�Ϊ����1���ٳ���©�� �ڼ���ƿ
��2��CaCO3+2HCl�TCaCl2+H2O+CO2�� BC
��©�����ˮ��û��©���ڣ���ֹˮ�м�ס�����Ҷ˵��齺�ܣ��ټ�����©�����ˮ�����©����ˮλ����©���⣬��һ��ʱ��ˮλ���ֲ��䣬��˵�����������ã������������ɣ�
CO2+Ca��OH��2�TCaCO3��+H2O
��3��2KMnO4
| ||
��4��Zn+H2SO4�TZnSO4+H2�� b b
������������Ŀ�ص㿼���ʵ������ȡ�����˼·�����⣬����ʵ������ȡ����ķ���װ�õ�ѡ������ɷ�Ӧ���״̬�ͷ�Ӧ�������������ģ�������ռ�װ�õ�ѡ������������ܽ��ԡ��ܶȵ����������������ģ����װ�ÿ������ռ����壬�����ſ������ռ��ܶȱȿ���С������ʱ������Ӷ̹ܽ�����֮�ӳ��ܽ�������ˮ���ռ�ʱ����Ӷ̹ܽ���

��ϰ��ϵ�д�
�����Ŀ