��Ŀ����
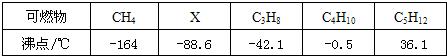
�±��Ǽ��ֿ�ȼ����ѹǿΪ101KPaʱ��ķе㣮����ش�| ��ȼ�� | CH4 | X | C3H8 | C4H10 | C5H12 |
| �е�/�� | -164 | -88.6 | -42.1 | -0.5 | -36.1 |
��2��X=
��3��������ȼ���Լ�ѹ������Һ������ѹ���أ�Һ��������������������ȼ�գ��ϱ��п�������ȼ�ϵ���
��4������ӱ����ҳ�һ������
���������ݷ�Ӧ��������P�������غ㶨�ɿ�����д��ѧ����ʽ��
���ݿ�ȼ��Ļ�ѧʽ��֪����ȼ��Ļ�ѧʽ��ͨʽΪ��CnH2n+2��n��ʾ̼ԭ�ӵĸ�����
ȼ���Լ�ѹ������Һ������ѹ��Һ��������������˵����ȼ�ϵķе㲻�ܸߣ�Ҳ���Ǻܵͣ�
�ɱ������ݿ�֪��һ������£���ȼ��ķе����ŷ�����̼ԭ�Ӹ��������Ӷ����ߣ�
���ݿ�ȼ��Ļ�ѧʽ��֪����ȼ��Ļ�ѧʽ��ͨʽΪ��CnH2n+2��n��ʾ̼ԭ�ӵĸ�����
ȼ���Լ�ѹ������Һ������ѹ��Һ��������������˵����ȼ�ϵķе㲻�ܸߣ�Ҳ���Ǻܵͣ�
�ɱ������ݿ�֪��һ������£���ȼ��ķе����ŷ�����̼ԭ�Ӹ��������Ӷ����ߣ�
����⣺��1������ȼ�յĻ�ѧ����ʽΪ��C3H8+5O2
3CO2+4H2O��
��2���ɱ������ʵĻ�ѧʽ�Ĺ��ɿ�֪��X��C2H6�����C2H6��
��3������ķе���-0.5�棬������Һ�������������������C4H10��
��4���ɱ������ݿ�֪����������£�һ��������̼ԭ�Ӹ���Խ�����ʵķе�Խ�ߣ������������£�һ��������̼ԭ�Ӹ���Խ�����ʵķе�Խ�ߣ�
| ||
��2���ɱ������ʵĻ�ѧʽ�Ĺ��ɿ�֪��X��C2H6�����C2H6��
��3������ķе���-0.5�棬������Һ�������������������C4H10��
��4���ɱ������ݿ�֪����������£�һ��������̼ԭ�Ӹ���Խ�����ʵķе�Խ�ߣ������������£�һ��������̼ԭ�Ӹ���Խ�����ʵķе�Խ�ߣ�
������������Ҫ���黯ѧ����ʽ����д�����������ṩ�������жϱ仯���ɵȷ����֪ʶ�����ʱҪ����Χ�Ʊ������ݽ��з������жϣ��Ӷ��ó���ȷ�Ľ��ۣ�

��ϰ��ϵ�д�
�����Ŀ
 ȼ������Ҫ����Դ��
ȼ������Ҫ����Դ����1�������ߡ�����ɹ���ʹ�й��������Ŀ��������һ�������ϵ�3He��3��ʾ���ԭ���������̲�����̽�µ�Ŀ��֮һ��̽��˾۱�ȼ��3He�ķֲ��������ϵĺ�Ԫ����Ҫ��4He��ʽ���ڣ���ԭ�ӵĹ���������3He��4He����ԭ�ӵ�
��2����Ȼ���dz��õ�ȼ�ϣ�������Ҫ�ɷֵ�������
��3���±��Ǽ��ֿ�ȼ����ѹǿΪ101kPaʱ�ķе㣮
| ��ȼ�� | CH4 | X | C3H8 | C4H10 | C5H12 |
| �е�/�� | -164 | -88.6 | -42.1 | -0.5 | 36.1 |
ȼ������Ҫ����Դ��
��1�������ߡ�����ɹ���ʹ�й��������Ŀ��������һ�������ϵ�3He��3��ʾ���ԭ���������̲�����̽�µ�Ŀ��֮һ��̽��˾۱�ȼ��3He�ķֲ��������ϵĺ�Ԫ����Ҫ��4He��ʽ���ڣ���ԭ�ӵĹ���������3He��4He����ԭ�ӵ� ����ͬ��
��2����Ȼ���dz��õ�ȼ�ϣ�������Ҫ�ɷֵ������� ��ȼ�յĻ�ѧ����ʽ�� ��
��3���±��Ǽ��ֿ�ȼ����ѹǿΪ101kPaʱ�ķе㣮
������ȼ���Լ�ѹ������Һ������ѹ���أ�Һ��������������������ȼ�գ��ϱ��п�������ȼ�ϵ��� �������ϱ��п�ȼ����ӽṹ�ϵĹ��ɣ��Ʋ�X�Ļ�ѧʽΪ �������㻹�ܴӱ����ܽ����һ�������� ��
��1�������ߡ�����ɹ���ʹ�й��������Ŀ��������һ�������ϵ�3He��3��ʾ���ԭ���������̲�����̽�µ�Ŀ��֮һ��̽��˾۱�ȼ��3He�ķֲ��������ϵĺ�Ԫ����Ҫ��4He��ʽ���ڣ���ԭ�ӵĹ���������3He��4He����ԭ�ӵ�
��2����Ȼ���dz��õ�ȼ�ϣ�������Ҫ�ɷֵ�������
��3���±��Ǽ��ֿ�ȼ����ѹǿΪ101kPaʱ�ķе㣮
| ��ȼ�� | CH4 | X | C3H8 | C4H10 | C5H12 |
| �е�/�� | -164 | -88.6 | -42.1 | -0.5 | 36.1 |