��Ŀ����
��2013?˳����һģ��̼�����ǻ�ѧ���������Ӵ�ļ��壬̼���仯�������������������Ź㷺Ӧ�ã�
��1��ͼ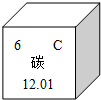 ΪԪ�����ڱ����й�̼Ԫ�ص���Ϣ��̼Ԫ��ԭ�Ӻ��ڵ�������Ϊ
ΪԪ�����ڱ����й�̼Ԫ�ص���Ϣ��̼Ԫ��ԭ�Ӻ��ڵ�������Ϊ
��2����̼�Ļ����ﱻ��Ϊ�л�������л��߷��Ӳ���Ӧ�ù㷺��������Ʒ�����úϳ��л��߷��Ӳ��ϵ���

��3��������̼��ѭ�����ڵ���������Ҫ��Ӱ�죮������̼��Ҫ��Դ�ڻ�ʯȼ�ϵ�ȼ�գ���ʯȼ�ϰ�����Ȼ����ú��������Ȼ����ȫȼ�յĻ�ѧ����ʽΪ
��4����ҵ���á�̼����������CO��CO2��������е�CO2��������CO���������������ͼ��ʾ����������������δ�������
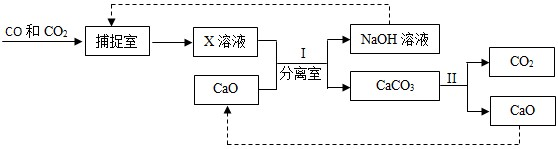
�ٷ�ӦII�������CO2���Ƴɸɱ����ɱ�������
�ڷ������н��еIJ�����
�������йظò����̵������У�����ȷ����
A��������CO2���Ʊ�����������Ʒ�����������������ŷ�
B���������ҡ��еķ�ӦҪ���մ�����
C�����������У�ֻ��һ�����ʿ�ѭ������
D���������Ĵ��Ǹò������д���������⣮
��1��ͼ
 ΪԪ�����ڱ����й�̼Ԫ�ص���Ϣ��̼Ԫ��ԭ�Ӻ��ڵ�������Ϊ
ΪԪ�����ڱ����й�̼Ԫ�ص���Ϣ��̼Ԫ��ԭ�Ӻ��ڵ�������Ϊ6
6
����2����̼�Ļ����ﱻ��Ϊ�л�������л��߷��Ӳ���Ӧ�ù㷺��������Ʒ�����úϳ��л��߷��Ӳ��ϵ���
B
B
������ĸ��ţ���
��3��������̼��ѭ�����ڵ���������Ҫ��Ӱ�죮������̼��Ҫ��Դ�ڻ�ʯȼ�ϵ�ȼ�գ���ʯȼ�ϰ�����Ȼ����ú��������Ȼ����ȫȼ�յĻ�ѧ����ʽΪ
CH4+2O2
CO2+2H2O
| ||
CH4+2O2
CO2+2H2O
��
| ||
��4����ҵ���á�̼����������CO��CO2��������е�CO2��������CO���������������ͼ��ʾ����������������δ�������

�ٷ�ӦII�������CO2���Ƴɸɱ����ɱ�������
�˹�����
�˹�����
���ڷ������н��еIJ�����
����
����
���������йظò����̵������У�����ȷ����
BC
BC
������ĸ��ţ���A��������CO2���Ʊ�����������Ʒ�����������������ŷ�
B���������ҡ��еķ�ӦҪ���մ�����
C�����������У�ֻ��һ�����ʿ�ѭ������
D���������Ĵ��Ǹò������д���������⣮
��������1������Ԫ�����ڱ��ṩ����Ϣ��ԭ������=������������
��2�����ݳ������ϵķ�����з�����
��3����Ȼ������Ҫ�ɷ��Ǽ��飬����ȼ�����ɶ�����̼��ˮ��
��4���ٸ��ݸɱ����������Ƚ��з������ڸ��ݹ�Һ����ķ����������۸���������Ϣ�����жϣ�
��2�����ݳ������ϵķ�����з�����
��3����Ȼ������Ҫ�ɷ��Ǽ��飬����ȼ�����ɶ�����̼��ˮ��
��4���ٸ��ݸɱ����������Ƚ��з������ڸ��ݹ�Һ����ķ����������۸���������Ϣ�����жϣ�
����⣺��1������Ԫ�����ڱ��ṩ����Ϣ��֪��̼��ԭ������=����������=6������̼Ԫ�صĺ���������Ϊ6��
�ʴ�Ϊ��6��
��2��A����̩�������л��߷��Ӳ��ϣ��ʴ���
B���������ںϳ��л��߷��Ӳ��ϣ�����ȷ��
C����˿������Ȼ�߷��Ӳ��ϣ��ʴ���
D����������ںϽ𣬹ʴ���
��ѡB��
��3����Ȼ������Ҫ�ɷ��Ǽ��飬����ȼ�����ɶ�����̼��ˮ����ѧ��Ӧʽ�ǣ�CH4+2O2
CO2+2H20��
�ʴ�Ϊ��CH4+2O2
CO2+2H20��
��4������Ϊ�ɱ����������մ������ȣ����Ըɱ��������˹����꣬�ʴ�Ϊ���˹����ꣻ
�ڷ��������Թ������Һһ���ù��˵ķ������ʴ�Ϊ�����ˣ�
��A����Ϊ������̼���������ЧӦ����Ҫ����֮һ����˽�������CO2�Ʊ�����������Ʒ���ɼ��������������ŷţ���A˵����ȷ��
B���������ڵIJ����ǹ��ˣ����������������B˵������
C���ڷ�Ӧ�У�������̼���������ƶ�����ѭ�����ã���C˵������
D��ͨ������һϵ�еķ�Ӧ���Կ����ò�������һ��ȱ���Ƿ�Ӧ�������ܺĴ�����������Ĵ��Ǹò������д���������⣬��D˵����ȷ��
��ѡBC��
�ʴ�Ϊ��6��
��2��A����̩�������л��߷��Ӳ��ϣ��ʴ���
B���������ںϳ��л��߷��Ӳ��ϣ�����ȷ��
C����˿������Ȼ�߷��Ӳ��ϣ��ʴ���
D����������ںϽ𣬹ʴ���
��ѡB��
��3����Ȼ������Ҫ�ɷ��Ǽ��飬����ȼ�����ɶ�����̼��ˮ����ѧ��Ӧʽ�ǣ�CH4+2O2
| ||
�ʴ�Ϊ��CH4+2O2
| ||
��4������Ϊ�ɱ����������մ������ȣ����Ըɱ��������˹����꣬�ʴ�Ϊ���˹����ꣻ
�ڷ��������Թ������Һһ���ù��˵ķ������ʴ�Ϊ�����ˣ�
��A����Ϊ������̼���������ЧӦ����Ҫ����֮һ����˽�������CO2�Ʊ�����������Ʒ���ɼ��������������ŷţ���A˵����ȷ��
B���������ڵIJ����ǹ��ˣ����������������B˵������
C���ڷ�Ӧ�У�������̼���������ƶ�����ѭ�����ã���C˵������
D��ͨ������һϵ�еķ�Ӧ���Կ����ò�������һ��ȱ���Ƿ�Ӧ�������ܺĴ�����������Ĵ��Ǹò������д���������⣬��D˵����ȷ��
��ѡBC��
���������⿼��ѧ����Ԫ�����ڱ��ṩ����Ϣ���ṹʾ��ͼ�����������Ӧ�õ�������ͬʱ������һ����̼��������̼����ķ������ѶȲ���

��ϰ��ϵ�д�
 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
�����Ŀ
 ��2013?˳����һģ����ͼΪʵ������ȡ����ij��÷���װ�ã��ش��������⣺
��2013?˳����һģ����ͼΪʵ������ȡ����ij��÷���װ�ã��ش��������⣺ ��2013?˳����һģ����ѧС���ͬѧ����ͼ��ʾ��װ��̽��������̼�����ʺ���̽��������˼�������̽����
��2013?˳����һģ����ѧС���ͬѧ����ͼ��ʾ��װ��̽��������̼�����ʺ���̽��������˼�������̽����