��Ŀ����
������һ����ǿ�Ҵ̼�����ζ�����壬����ֱ���ŷŵ������У�ʵ�����ð�����ԭ����ͭ�ķ����ⶨͭ�����ԭ����������֪��2NH4Cl+Ca��OH��2 CaCl2+2NH3��+2H2O
CaCl2+2NH3��+2H2O2NH3+3CuO
 N2+3Cu+3H2O
N2+3Cu+3H2O2NH3+H2SO4=��NH4��2SO4
�Իش�
��1�����ѡ�òⶨ��Ӧ��CuO��������H2O������[m��CuO����m��H2O��]�����������������һ����ʵ�鷽����
���������ӵ�˳������ĸ��ű�ʾ���������ظ�ʹ�ã� ��
���г�����Cu�����ԭ�������ı���ʽ ��
�����������ʹ�ⶨ���ƫ����� ������ѡ����ղ���1����ȷ�𰸣�������ĸ�����д��
��a��CuOδȫ����ԭΪCu ��b��CuO�ܳ� ��c��CuO�л���Cu
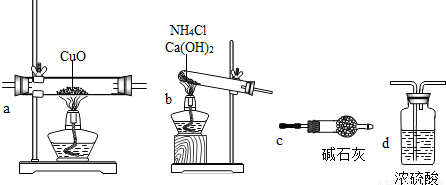
��2������Բ�����������װ�ã�����������ѡ�òⶨ����������
��a��m��Cu����m��CuO�� ��b��m��N2����m��H2O�� ��c��m��Cu����m��H2O�� ��d��m��NH3����m��H2O��
���𰸡���������1���ٸ��������Ҫ������������ͭ��ˮ�����������ǵ��������Ժ�Ũ���ᷴӦ�����Dz��ͼ�ʯ�ҷ�Ӧ������Ӧ���ü�ʯ��������ˮ������Ũ���������հ��������Ծݴ˴��⣻
�ڸ���ˮ������ͭ��������ϻ�ѧ����ʽ2NH3+3CuO N2+3Cu+3H2O���м��㼴�����ͭ�����ԭ��������
N2+3Cu+3H2O���м��㼴�����ͭ�����ԭ��������
�۸��ݢڼ���Ľ�����Է�����ʹ�ⶨ���ƫ������أ�
��2�����������װ���ص��������ó�����ѡ�õ����ʵ�������
����⣺��1���ٸ��������Ҫ������������ͭ��ˮ�����������ǵ��������Ժ�Ũ���ᷴӦ�����Dz��ͼ�ʯ�ҷ�Ӧ������Ӧ���ü�ʯ��������ˮ������Ũ���������հ�������������ȡ�����Ĺ�����Ҫ����ˮ������Ӧ���ȶԲ����İ������и����ѡ��cװ�ã�Ȼ�����ʵ�飬���Ϊ�˷�ֹ������Ⱦ����������Ҫ��Ũ����������β��������ȷ�IJ���˳��Ϊ��bcacd��
����ͭ�����ԭ������Ϊx
2NH3+3CuO N2+3Cu+3H2O
N2+3Cu+3H2O
3��x+16��54
m��CuO�� m��H2O��
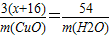
���x=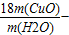 16
16
�۸��ݢڵĽ�����֪���������ͭû����ȫ����ԭ���ǻ�������������Ӧ�����ʶ��ᵼ�½�����ƫ�������a��CuOδȫ����ԭΪCu����������ˮ������ƫС�����Ե��¼�����ƫ��b����ͭ���ܳ������ȹ���ˮ�����������²��ˮ������ƫ��c�к���ͭ��ʹ������ˮ������ƫС�������ܹ�ʹ������ƫ���Ϊa��c��
��2�����ݻ�ѧ����ʽ2NH3+3CuO N2+3Cu+3H2O����֪�������ǿ��Բ�õ�����Ϊ��ͭ��������ˮ������������ͭ�����������ǵ����Ͱ������������ɲⶨ����ֻҪ��ͭ��ˮ������ͭ������������ϼ��ɣ�
N2+3Cu+3H2O����֪�������ǿ��Բ�õ�����Ϊ��ͭ��������ˮ������������ͭ�����������ǵ����Ͱ������������ɲⶨ����ֻҪ��ͭ��ˮ������ͭ������������ϼ��ɣ�
�ʴ�Ϊ����1����bcacd��
��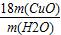 -16��
-16��
��ac��
��2��ac��
������ע����������������֪��Ũ����ͼ�ʯ�ҵ����ã�Ϊ���ô���������İ�����ԭ����ͭ����ͨ���ⶨ����ˮ����������ͭ�����ԭ��������Ҫע���ȳ�ȥ�����е����ʣ�����ȥˮ��
�ڸ���ˮ������ͭ��������ϻ�ѧ����ʽ2NH3+3CuO
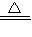 N2+3Cu+3H2O���м��㼴�����ͭ�����ԭ��������
N2+3Cu+3H2O���м��㼴�����ͭ�����ԭ���������۸��ݢڼ���Ľ�����Է�����ʹ�ⶨ���ƫ������أ�
��2�����������װ���ص��������ó�����ѡ�õ����ʵ�������
����⣺��1���ٸ��������Ҫ������������ͭ��ˮ�����������ǵ��������Ժ�Ũ���ᷴӦ�����Dz��ͼ�ʯ�ҷ�Ӧ������Ӧ���ü�ʯ��������ˮ������Ũ���������հ�������������ȡ�����Ĺ�����Ҫ����ˮ������Ӧ���ȶԲ����İ������и����ѡ��cװ�ã�Ȼ�����ʵ�飬���Ϊ�˷�ֹ������Ⱦ����������Ҫ��Ũ����������β��������ȷ�IJ���˳��Ϊ��bcacd��
����ͭ�����ԭ������Ϊx
2NH3+3CuO
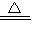 N2+3Cu+3H2O
N2+3Cu+3H2O3��x+16��54
m��CuO�� m��H2O��
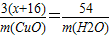
���x=
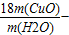 16
16�۸��ݢڵĽ�����֪���������ͭû����ȫ����ԭ���ǻ�������������Ӧ�����ʶ��ᵼ�½�����ƫ�������a��CuOδȫ����ԭΪCu����������ˮ������ƫС�����Ե��¼�����ƫ��b����ͭ���ܳ������ȹ���ˮ�����������²��ˮ������ƫ��c�к���ͭ��ʹ������ˮ������ƫС�������ܹ�ʹ������ƫ���Ϊa��c��
��2�����ݻ�ѧ����ʽ2NH3+3CuO
 N2+3Cu+3H2O����֪�������ǿ��Բ�õ�����Ϊ��ͭ��������ˮ������������ͭ�����������ǵ����Ͱ������������ɲⶨ����ֻҪ��ͭ��ˮ������ͭ������������ϼ��ɣ�
N2+3Cu+3H2O����֪�������ǿ��Բ�õ�����Ϊ��ͭ��������ˮ������������ͭ�����������ǵ����Ͱ������������ɲⶨ����ֻҪ��ͭ��ˮ������ͭ������������ϼ��ɣ��ʴ�Ϊ����1����bcacd��
��
 -16��
-16����ac��
��2��ac��
������ע����������������֪��Ũ����ͼ�ʯ�ҵ����ã�Ϊ���ô���������İ�����ԭ����ͭ����ͨ���ⶨ����ˮ����������ͭ�����ԭ��������Ҫע���ȳ�ȥ�����е����ʣ�����ȥˮ��

��ϰ��ϵ�д�
�����Ŀ
 С������ڼ䵽ũ��ʩ��ʱ�����������ڵ��ϵ�̼泥�̼����淋ļ�ƣ�������������ºܿ���ʧ�ˣ�ͬʱ��Ũ�ҵĴ̼�����ζ�����ܺ��棬��У���ͬѧ�ǽ���̽��������һͬ���룺
С������ڼ䵽ũ��ʩ��ʱ�����������ڵ��ϵ�̼泥�̼����淋ļ�ƣ�������������ºܿ���ʧ�ˣ�ͬʱ��Ũ�ҵĴ̼�����ζ�����ܺ��棬��У���ͬѧ�ǽ���̽��������һͬ���룺
��������⡿̼����狀ܿ���ʧ����һ�����������仯���ǻ�ѧ�仯��
���������ϡ�
��1��������̼���һ����ζ��������ˮ�İ�ɫ���壮���ص����ڵ��¡�����������£����ȶ����ڣ����ǣ����¶��Ըߣ�10�����ϣ���ʪ���Դ�̼茶�����ʧ��̼鱗���ʹʪ��ķ�̪��ֽ���ɫ��
��2��������̼������ʹ�����ʯ��ˮ����ǣ������ڼ��������̼���壮̼�����Ҳ��ʹ����ʯ��ˮ����ǣ�
��3��������ʹʪ��ķ�̪��ֽ���ɫ�������ڼ��鰱����NH3����
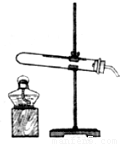
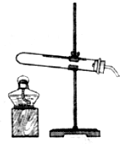
���ռ�֤�ݡ���1������ͼ��ʾ��װ��ʵ��װ�ã���װ������˳����________
��2�����װ�õ������ԣ���鷽����________
��3������ʵ�飺
| ����ʵ���顡������ | ����ʵ���顡�֡��� | ����ʵ���顡�ᡡ�� |
| �����Թ��м�������̼立�ĩ�����ȣ���һ�״ɰ���ڵ��ܿڵ��·� | ||
| �ڽ�ʪ��ķ�̪��ֽ��̼什Ӵ� | ����̪��ֽû����ɫ�仯 | ̼鱗���ʹ��̪��ֽ��� |
| �۽�ʪ��ķ�̪��ֽ�����Թܿڴ� |
����˼�뽻������1��ʵ��ʱС��ͬѧ���Թ��ڱڸ���̼立�ĩ����Ľ�����________��С��ͬѧ���Թ������ˣ�����Ϊԭ������ǣ�дһ����________��
��2���е�ͬѧ��Ϊ����ʵ�鲻��Ҫ��̼��ܷ���ʪ��ķ�̪��ֽ���õ�ʵ�飬��ͬ����Ϊʲô��________��
��3�������������ζʱ�е�ͬѧֱ�Ӱѱ��Ӵյ��Թܿڴ�������ܵ��˱Ƚ�ǿ�ҵĴ̼�������Ϊ��ȷ��������________��������μ���ʵ����������������ǿ�Ҵ̼�����Ľ�����________
��4���е�ͬѧ�ѵ�����ͨ�뵽�����ʯ��ˮ��ʯ��ˮ����ǣ�����Ϊ�ɴ�Ҳ��˵�������˶�����̼�������˻�ѧ�仯������Ϊ���ɳ����Ϊʲô��
________
��5����������ʵ�飬����Ϊ̼����炙����ڴ��ʱӦע���������________��
С������ڼ䵽ũ��ʩ��ʱ�����������ڵ��ϵ�̼泥�̼����淋ļ�ƣ�������������ºܿ���ʧ�ˣ�ͬʱ��Ũ�ҵĴ̼�����ζ�����ܺ��棬��У���ͬѧ�ǽ���̽��������һͬ���룺
��������⡿̼����狀ܿ���ʧ����һ�����������仯���ǻ�ѧ�仯��
���������ϡ�
��1��������̼���һ����ζ��������ˮ�İ�ɫ���壮���ص����ڵ��¡�����������£����ȶ����ڣ����ǣ����¶��Ըߣ�10�����ϣ���ʪ���Դ�̼茶�����ʧ��̼鱗���ʹʪ��ķ�̪��ֽ���ɫ��
��2��������̼������ʹ�����ʯ��ˮ����ǣ������ڼ��������̼���壮̼�����Ҳ��ʹ����ʯ��ˮ����ǣ�
��3��������ʹʪ��ķ�̪��ֽ���ɫ�������ڼ��鰱����NH3����
���ռ�֤�ݡ���1������ͼ��ʾ��װ��ʵ��װ�ã���װ������˳����______
��2�����װ�õ������ԣ���鷽����______
��3������ʵ�飺
����ý��ۡ�̼�������ʧ�ˣ������������ʣ������˻�ѧ�仯��
����˼�뽻������1��ʵ��ʱС��ͬѧ���Թ��ڱڸ���̼立�ĩ����Ľ�����______��С��ͬѧ���Թ������ˣ�����Ϊԭ������ǣ�дһ����______��
��2���е�ͬѧ��Ϊ����ʵ�鲻��Ҫ��̼��ܷ���ʪ��ķ�̪��ֽ���õ�ʵ�飬��ͬ����Ϊʲô��______��
��3�������������ζʱ�е�ͬѧֱ�Ӱѱ��Ӵյ��Թܿڴ�������ܵ��˱Ƚ�ǿ�ҵĴ̼�������Ϊ��ȷ��������______��������μ���ʵ����������������ǿ�Ҵ̼�����Ľ�����______
��4���е�ͬѧ�ѵ�����ͨ�뵽�����ʯ��ˮ��ʯ��ˮ����ǣ�����Ϊ�ɴ�Ҳ��˵�������˶�����̼�������˻�ѧ�仯������Ϊ���ɳ����Ϊʲô��
______
��5����������ʵ�飬����Ϊ̼����炙����ڴ��ʱӦע���������______��
��������⡿̼����狀ܿ���ʧ����һ�����������仯���ǻ�ѧ�仯��
���������ϡ�
��1��������̼���һ����ζ��������ˮ�İ�ɫ���壮���ص����ڵ��¡�����������£����ȶ����ڣ����ǣ����¶��Ըߣ�10�����ϣ���ʪ���Դ�̼茶�����ʧ��̼鱗���ʹʪ��ķ�̪��ֽ���ɫ��
��2��������̼������ʹ�����ʯ��ˮ����ǣ������ڼ��������̼���壮̼�����Ҳ��ʹ����ʯ��ˮ����ǣ�
��3��������ʹʪ��ķ�̪��ֽ���ɫ�������ڼ��鰱����NH3����
���ռ�֤�ݡ���1������ͼ��ʾ��װ��ʵ��װ�ã���װ������˳����______
��2�����װ�õ������ԣ���鷽����______
��3������ʵ�飺
| ʵ �� �� �� | ʵ �� �� �� | ʵ �� �� �� |
| �����Թ��м�������̼立�ĩ�����ȣ���һ�״ɰ���ڵ��ܿڵ��·� | ||
| �ڽ�ʪ��ķ�̪��ֽ��̼什Ӵ� | ��̪��ֽû����ɫ�仯 | ̼鱗���ʹ��̪��ֽ��� |
| �۽�ʪ��ķ�̪��ֽ�����Թܿڴ� |
����˼�뽻������1��ʵ��ʱС��ͬѧ���Թ��ڱڸ���̼立�ĩ����Ľ�����______��С��ͬѧ���Թ������ˣ�����Ϊԭ������ǣ�дһ����______��
��2���е�ͬѧ��Ϊ����ʵ�鲻��Ҫ��̼��ܷ���ʪ��ķ�̪��ֽ���õ�ʵ�飬��ͬ����Ϊʲô��______��
��3�������������ζʱ�е�ͬѧֱ�Ӱѱ��Ӵյ��Թܿڴ�������ܵ��˱Ƚ�ǿ�ҵĴ̼�������Ϊ��ȷ��������______��������μ���ʵ����������������ǿ�Ҵ̼�����Ľ�����______
��4���е�ͬѧ�ѵ�����ͨ�뵽�����ʯ��ˮ��ʯ��ˮ����ǣ�����Ϊ�ɴ�Ҳ��˵�������˶�����̼�������˻�ѧ�仯������Ϊ���ɳ����Ϊʲô��
______
��5����������ʵ�飬����Ϊ̼����炙����ڴ��ʱӦע���������______��

 С������ڼ䵽ũ��ʩ��ʱ�����������ڵ��ϵ�̼泥�̼����淋ļ�ƣ�������������ºܿ���ʧ�ˣ�ͬʱ��Ũ�ҵĴ̼�����ζ�����ܺ��棬��У���ͬѧ�ǽ���̽��������һͬ���룺
С������ڼ䵽ũ��ʩ��ʱ�����������ڵ��ϵ�̼泥�̼����淋ļ�ƣ�������������ºܿ���ʧ�ˣ�ͬʱ��Ũ�ҵĴ̼�����ζ�����ܺ��棬��У���ͬѧ�ǽ���̽��������һͬ���룺