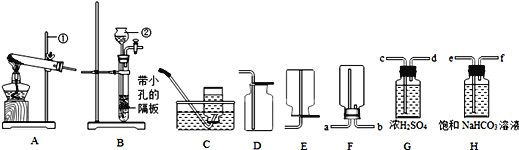
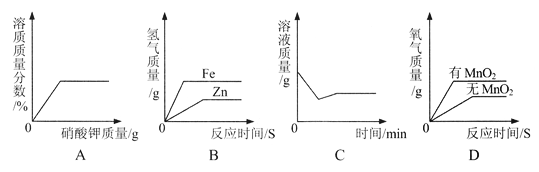
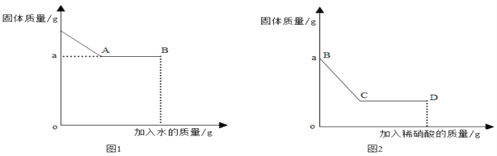
��Ŀ����
����Ŀ���������������Ĵ��������������⣬ij�����������������������д������ۺ����õIJ���������ͼ��
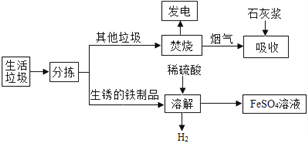
����1���������ղ����������к���SO2��HCl�����塣
����2��+2�۵���Ԫ�����ױ������е�����������
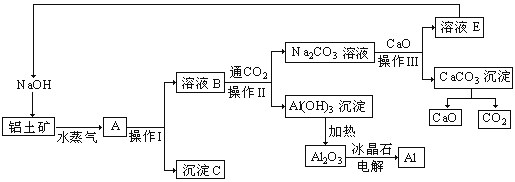
(1)���糧�������������ղ�����________��ת��Ϊ���ܡ�
(2)���ܽⲽ���У�������H2�ķ�Ӧ�⣬��������������Ӧ��_______(д����ѧ����ʽ)��Fe+Fe2(SO4)3=3FeSO4��
(3)�����õ�����������Һ�ڵ�������������Ũ����_________(���������)�����ˣ��õ�FeSO4��7H2O�����е�����������________��
(4)���ղ����У�ʯ�ҽ���������_________����1000g�����к���1.6g��������������Щ��������������Ҫ_______________��Ca(OH)2��
���𰸡� �� Fe2O3+3H2SO4==Fe2(SO4)3+3H2O ���½ᾧ ������ ��ֹ+2�۵���Ԫ�ر������е��������� ��ȥ�����к��е�SO2��HCl���к�����
����������1���������տ��Էų����������з��磬�ù����н�����ת��Ϊ������
��2���ܽ������������ϡ����Ҳ�����˷�Ӧ��������������ˮ����Ӧ����ʽΪFe2O3+3H2SO4==Fe2(SO4)3+3H2O��
��3�������������ܽ�����¶ȵ����߶����ߣ����ý��½ᾧ�ķ����ᴿ����������2���������ױ�����Ϊ3�������ʸù�����Ҫ�ڵ����Ļ����½��У�
(4)���ղ����У�ʯ�ҽ��������dz�ȥ�����к��е�SO2��HCl���к����壻
������1.6g�Ķ�������������Ҫ�������Ƶ�����Ϊx
SO2+Ca(OH)2=CaSO3![]() +H2O
+H2O
64 74
1.6g x
![]() =
=![]() �����x=1.85g
�����x=1.85g

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�����Ŀ��ȥ�굳��ʮ�Ŵ����ʹ����һ�ֺ�̼��Ƶġ�ʯͷֽ����Ϊ�ⶨ����̼��Ƶĺ���������С���ͬѧ����ȡ50g��ֽ��Ʒ���ֱ����5ֻ�ձ��в���ţ��ٷֱ���5ֻ�ձ��м���Ũ����ͬ��ϡ�������ʵ�顣ʵ�����ݼ��±�(����ֽ���е������ɷּȲ�����ˮ��Ҳ�������ᷴӦ)��
�ձ��� | �ձ��� | �ձ��� | �ձ��� | �ձ��� | |
������Ʒ������/g | 10 | 10 | 10 | 10 | 10 |
����ϡ���������/g | 10 | 20 | 30 | 40 | 50 |
��ַ�Ӧ���������������/g | 0.88 | 1.76 | 2.64 | m | 3.52 |
��1������m��ֵΪ___________��
��2������Ʒ��̼��Ƶ���������___________��
��3������Ʒ��̼��ƺ�����պ���ȫ��Ӧ��õ���Һ���ʵ��������������������С�����ڶ�λ��___