��Ŀ����
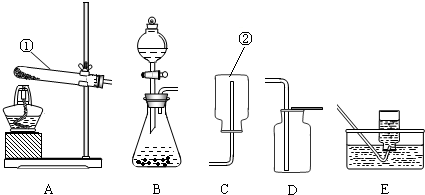
ʵ������ȡ����ij���װ����ͼ��ʾ��������ѧ��֪ʶ�ش���������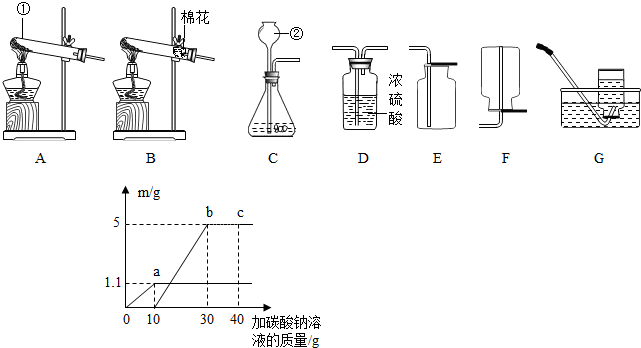
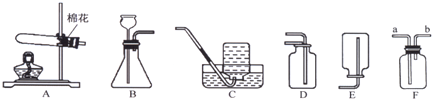
��1��д��װ���б�����������ƣ���
��2��ʵ�����ø��������ȡ��������ѡ�õķ���װ����
��3��д��ʵ�����ô���ʯ��ϡ������ȡ������̼�Ļ�ѧ����ʽ��
���ݴ�ѡ����ͼ��
��4��С����ʵ������ȡ������̼��ķ�Һ���ã�ȡ�ϲ���Һ50g����������μ�����������Ϊ26.5%��̼������Һ��������ʵ���õ����ݻ��ͼ������������m��ʵ��õ��ij�����������������������ʾ���Ǽ���̼������Һ���������Լ��㣺
��50g��Һ�к��Ȼ��Ƶ�������
��b���ʾ����Һ���Ȼ��Ƶ�����������

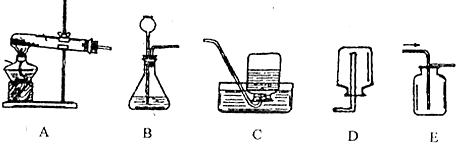
��1��д��װ���б�����������ƣ���
�Թ�
�Թ�
��������©��
����©��
����2��ʵ�����ø��������ȡ��������ѡ�õķ���װ����
B
B
������ĸ����д���÷�Ӧ�Ļ�ѧ����ʽ��2KMnO4
K2MnO4+MnO2+O2��
| ||
2KMnO4
K2MnO4+MnO2+O2��
��
| ||
��3��д��ʵ�����ô���ʯ��ϡ������ȡ������̼�Ļ�ѧ����ʽ��
CaCO3+2HCl�TCaCl2+H2O+CO2��
CaCO3+2HCl�TCaCl2+H2O+CO2��
���ݴ�ѡ����ͼ��
CDE
CDE
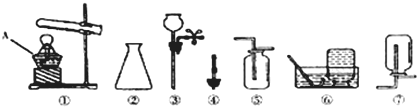
������ĸ����װһ����ȡ���������̼��װ�ã���4��С����ʵ������ȡ������̼��ķ�Һ���ã�ȡ�ϲ���Һ50g����������μ�����������Ϊ26.5%��̼������Һ��������ʵ���õ����ݻ��ͼ������������m��ʵ��õ��ij�����������������������ʾ���Ǽ���̼������Һ���������Լ��㣺
��50g��Һ�к��Ȼ��Ƶ�������
��b���ʾ����Һ���Ȼ��Ƶ�����������
��������1���������ջ�ѧ�������������ƺ���;��
��2�����ݷ�Ӧ���״̬�ͷ�Ӧ����ѡ����װ�ã�������ؼ��ȷֽ��������ء��������̺�������
��3������ʯ��ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼������װ�õ��ص��ǹ�Һ�����ͣ���Ũ�������������ſ������ռ�����Ķ�����̼��
��4����ͼ���֪��Һ��������ʣ�࣬����ͷ10��̼���������ᷴӦ������1.1�˶�����̼�����ᷴӦû�ˣ��Ȼ�����̼���Ʒ�Ӧ������5�˳������ݴ˾Ϳ�������Ȼ��ƺ����ɵ��Ȼ��Ƶ������ˣ�
��2�����ݷ�Ӧ���״̬�ͷ�Ӧ����ѡ����װ�ã�������ؼ��ȷֽ��������ء��������̺�������
��3������ʯ��ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼������װ�õ��ص��ǹ�Һ�����ͣ���Ũ�������������ſ������ռ�����Ķ�����̼��
��4����ͼ���֪��Һ��������ʣ�࣬����ͷ10��̼���������ᷴӦ������1.1�˶�����̼�����ᷴӦû�ˣ��Ȼ�����̼���Ʒ�Ӧ������5�˳������ݴ˾Ϳ�������Ȼ��ƺ����ɵ��Ȼ��Ƶ������ˣ�
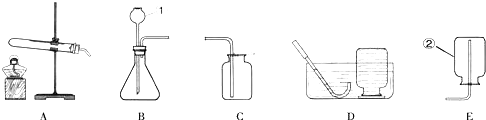
����⣺��1�������Թܣ����dz���©����
��2��������ؼ��ȷֽ��������ء��������̺��������ʷ���װ�õ��ص��ǹ�������͵�Bװ�ã���Ӧ�ķ���ʽΪ��2KMnO4
K2MnO4+MnO2+O2����
��3������ʯ��ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�ķ���ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2��������װ�õ��ص��ǹ�Һ�����͵�Cװ�ã���Ũ������������̼���ܶȱȿ������������ſ������ռ�����ѡ��CDE��װһ����ȡ���������̼��װ�ã�
��4���⣺�����Ȼ��Ƶ�����ΪX
CaCl2+Na2CO3=CaCO3��+2NaCl
111 100
X 5g
=
���X=5.55��
��CaCl2+Na2CO3=CaCO3��+2NaCl
Na2CO3+2HCl�T2NaCl+H2O+CO2��
���������غ㶨�ɣ�30g̼������Һ�е���Ԫ�����������շ�Ӧ�����Һ�е��Ȼ��Ƶ���Ԫ����������ȵģ�����30g̼������Һ�е���Ԫ�ص�����Ϊ30��26.5%��
��100%=3.45g��
��b��ʱ�Ȼ��Ƶ�����Ϊ3.45g/��
��100%��=8.775g
��ʱ��Һ������Ϊ��50g+30g-5g-1.1g=73.9g
�Ȼ��Ƶ���������Ϊ
��100%=11.9%
��50g��Һ�к��Ȼ���5.55g��b���ʾ����Һ���Ȼ��Ƶ���������Ϊ11.9%��
�ʴ�Ϊ����1���Թܣ�����©����
��2��B�� 2KMnO4
K2MnO4+MnO2+O2����
��3��CaCO3+2HCl�TCaCl2+H2O+CO2����CDE��
��4���⣺�����Ȼ��Ƶ�����ΪX
CaCl2+Na2CO3=CaCO3��+2NaCl
111 100
X 5g
=
���X=5.55��
��CaCl2+Na2CO3=CaCO3��+2NaCl
Na2CO3+2HCl�T2NaCl+H2O+CO2��
���������غ㶨�ɣ�30g̼������Һ�е���Ԫ�����������շ�Ӧ�����Һ�е��Ȼ��Ƶ���Ԫ����������ȵģ�����30g̼������Һ�е���Ԫ�ص�����Ϊ30��26.5%��
��100%=3.45g��
��b��ʱ�Ȼ��Ƶ�����Ϊ3.45g/��
��100%��=8.775g
��ʱ��Һ������Ϊ��50g+30g-5g-1.1g=73.9g
�Ȼ��Ƶ���������Ϊ
��100%=11.9%
��50g��Һ�к��Ȼ���5.55g��b���ʾ����Һ���Ȼ��Ƶ���������Ϊ11.9%��
��2��������ؼ��ȷֽ��������ء��������̺��������ʷ���װ�õ��ص��ǹ�������͵�Bװ�ã���Ӧ�ķ���ʽΪ��2KMnO4
| ||
��3������ʯ��ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�ķ���ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2��������װ�õ��ص��ǹ�Һ�����͵�Cװ�ã���Ũ������������̼���ܶȱȿ������������ſ������ռ�����ѡ��CDE��װһ����ȡ���������̼��װ�ã�
��4���⣺�����Ȼ��Ƶ�����ΪX
CaCl2+Na2CO3=CaCO3��+2NaCl
111 100
X 5g
| 111 |
| 100 |
| X |
| 5g |
���X=5.55��
��CaCl2+Na2CO3=CaCO3��+2NaCl
Na2CO3+2HCl�T2NaCl+H2O+CO2��
���������غ㶨�ɣ�30g̼������Һ�е���Ԫ�����������շ�Ӧ�����Һ�е��Ȼ��Ƶ���Ԫ����������ȵģ�����30g̼������Һ�е���Ԫ�ص�����Ϊ30��26.5%��
| 46 |
| 106 |
��b��ʱ�Ȼ��Ƶ�����Ϊ3.45g/��
| 23 |
| 58.5 |
��ʱ��Һ������Ϊ��50g+30g-5g-1.1g=73.9g
�Ȼ��Ƶ���������Ϊ
| 8.775g |
| 73.9g |
��50g��Һ�к��Ȼ���5.55g��b���ʾ����Һ���Ȼ��Ƶ���������Ϊ11.9%��
�ʴ�Ϊ����1���Թܣ�����©����
��2��B�� 2KMnO4
| ||
��3��CaCO3+2HCl�TCaCl2+H2O+CO2����CDE��
��4���⣺�����Ȼ��Ƶ�����ΪX
CaCl2+Na2CO3=CaCO3��+2NaCl
111 100
X 5g
| 111 |
| 100 |
| X |
| 5g |
���X=5.55��
��CaCl2+Na2CO3=CaCO3��+2NaCl
Na2CO3+2HCl�T2NaCl+H2O+CO2��
���������غ㶨�ɣ�30g̼������Һ�е���Ԫ�����������շ�Ӧ�����Һ�е��Ȼ��Ƶ���Ԫ����������ȵģ�����30g̼������Һ�е���Ԫ�ص�����Ϊ30��26.5%��
| 46 |
| 106 |
��b��ʱ�Ȼ��Ƶ�����Ϊ3.45g/��
| 23 |
| 58.5 |
��ʱ��Һ������Ϊ��50g+30g-5g-1.1g=73.9g
�Ȼ��Ƶ���������Ϊ
| 8.775g |
| 73.9g |
��50g��Һ�к��Ȼ���5.55g��b���ʾ����Һ���Ȼ��Ƶ���������Ϊ11.9%��
���������⿼���˳����������ȡ���ռ������ݻ�ѧ����ʽ�ļ��㣬�ڼ����йؼ��Ǹ������������֪��������ؽ��⣮

��ϰ��ϵ�д�
�����Ŀ