��Ŀ����
���÷��ӵĹ۵������������
��1���߹������ŵ�����
��2��50mLˮ��50mL�ƾ�������С��100mL
��3������������Һ�м�������������������ɣ���ˮ�м����������������������
��4�����ˮ���Ƶ��������������䷴Ӧ��ʵ����
��1���߹������ŵ�����
�����Dz����˶���
�����Dz����˶���
����2��50mLˮ��50mL�ƾ�������С��100mL
���Ӽ��м��
���Ӽ��м��
����3������������Һ�м�������������������ɣ���ˮ�м����������������������
�������ʵķ��Ӳ�ͬ�����ʲ�ͬ
�������ʵķ��Ӳ�ͬ�����ʲ�ͬ
����4�����ˮ���Ƶ��������������䷴Ӧ��ʵ����
���ӷ��ѳ�ԭ�ӣ�ԭ��������ϳ��µķ���
���ӷ��ѳ�ԭ�ӣ�ԭ��������ϳ��µķ���
�����������ݷ��ӵĻ��������������������������С������֮���м�����������ڲ����˶��ģ�ͬ�����ʵķ���������ͬ����ͬ���ʵķ������ʲ�ͬ��������з����жϼ��ɣ�
����⣺��1�����ڷ����Dz����˶��ģ�����ķ���ͨ���˶���ɢ����Χ�Ŀ����У����ԣ��߹������ŵ����㣻
��2�����ڷ��Ӽ��м������50mLˮ��50mL�ƾ����ʱ�������ռ���˼�������ԣ������С��100mL��
��3�����ڹ���������ˮ�ķ��ӹ��ɲ�ͬ�����ʲ�ͬ�����ԣ�����������Һ�м�������������������ɣ���ˮ�м���������������������ɣ�
��4�����ˮʱ��ˮ���ӷֽ�Ϊ��ԭ�Ӻ���ԭ�ӣ�ÿ������ԭ�ӽ�ϳ�һ������ӣ�������������γ�������ÿ������ԭ�ӽ�ϳ�һ�������ӣ��������������γ����������ԣ��ɵó���ѧ��Ӧ��ʵ���ǣ����ӷ��ѳ�ԭ�ӣ�ԭ��������ϳ��µķ��ӣ�
�ʴ�Ϊ����1�������Dz����˶��ģ���2�����Ӽ��м������3���������ʵķ��Ӳ�ͬ�����ʲ�ͬ����4�����ӷ��ѳ�ԭ�ӣ�ԭ��������ϳ��µķ��ӣ�
��2�����ڷ��Ӽ��м������50mLˮ��50mL�ƾ����ʱ�������ռ���˼�������ԣ������С��100mL��
��3�����ڹ���������ˮ�ķ��ӹ��ɲ�ͬ�����ʲ�ͬ�����ԣ�����������Һ�м�������������������ɣ���ˮ�м���������������������ɣ�
��4�����ˮʱ��ˮ���ӷֽ�Ϊ��ԭ�Ӻ���ԭ�ӣ�ÿ������ԭ�ӽ�ϳ�һ������ӣ�������������γ�������ÿ������ԭ�ӽ�ϳ�һ�������ӣ��������������γ����������ԣ��ɵó���ѧ��Ӧ��ʵ���ǣ����ӷ��ѳ�ԭ�ӣ�ԭ��������ϳ��µķ��ӣ�
�ʴ�Ϊ����1�������Dz����˶��ģ���2�����Ӽ��м������3���������ʵķ��Ӳ�ͬ�����ʲ�ͬ����4�����ӷ��ѳ�ԭ�ӣ�ԭ��������ϳ��µķ��ӣ�
�����������ѶȲ����������÷��ӵĻ������ʷ����ͽ������ķ����ǽ�������Ĺؼ���

��ϰ��ϵ�д�
 â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
�����Ŀ


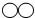



 ����̫���ܡ����ܵ�������ԴΪ��������������ü�ޡ���ȼ������ʹ�ù����з�����Ӧ�Ļ�ѧ����ʽΪ ���ӻ����ĽǶ���Ϊ��ʹ����ȼ��������ͻ�����ŵ��� ��
����̫���ܡ����ܵ�������ԴΪ��������������ü�ޡ���ȼ������ʹ�ù����з�����Ӧ�Ļ�ѧ����ʽΪ ���ӻ����ĽǶ���Ϊ��ʹ����ȼ��������ͻ�����ŵ��� ��