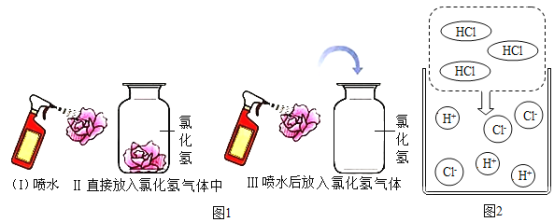
��Ŀ����
����Ŀ��������ͼ��ʾ��ʵ��װ�ã�
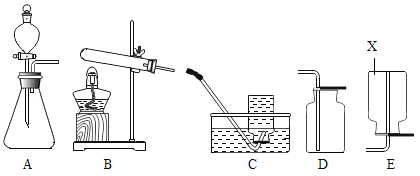
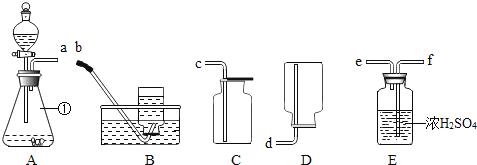
��1��д���������X�����ƣ�_____________��
��2��Aװ�ÿ������ƶ�����̼�Ļ�ѧ����ʽΪ��_________________���÷���װ�û�����������ȡ��������________��д��Ӧ�Ļ�ѧ����ʽ��___________________��
��3����Dװ���ռ�������̼����ʱ�����������___________________��
��4��ʵ���������������������ü�����ˮ���������ʯ�ҵĹ��������Ƶá��Ƽ������ķ���װ��Ӧѡ��_______������ĸ��װ�ã��ռ���������ѡ��C��Eװ�ã��ɴ��ƶϼ���������е�����������_____________��
���𰸡�����ƿ CaCO3+2HCl=CaCl2+H2O+CO2![]() O2
O2 ![]() ��ȼ�ŵ�ľ�����ڼ���ƿ�� B ��������ˮ���ܶȱȿ���С
��ȼ�ŵ�ľ�����ڼ���ƿ�� B ��������ˮ���ܶȱȿ���С
��������
��1������X������Ϊ������ƿ��
��2���ƶ�����̼��̼��ƺ�ϡ���ᷴӦ�����Ȼ��ƺͶ�����̼����Ӧ�Ļ�ѧ����ʽΪ��![]() ���÷���װ�û�����������ȡ���������������������̴��������ⷴӦ����ˮ����������Ӧ�Ļ�ѧ����ʽΪ��
���÷���װ�û�����������ȡ���������������������̴��������ⷴӦ����ˮ����������Ӧ�Ļ�ѧ����ʽΪ��![]() ��
��
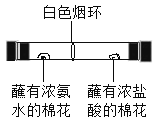
��3����Dװ���ռ�������̼����ʱ���������ԣ���ȼ�ŵ�ľ�����ڼ���ƿ�ڣ�ľ��Ϩ��������
��4��������ˮ���������ʯ�ҵĹ��������Ƶü��飬���ڹ�-�̻�ϼ����ͣ�����װ��Ӧѡ��Bװ�ã��ռ���������ѡ��C��Eװ�ã��ɴ��ƶϼ���������е����������Dz�������ˮ���ܶȱȿ���С��

����Ŀ��ʵ��������һƿ���õ���ɫ��Һ���ܲ��������ȱ�ı�ǩ��ֻʣ����Na������10%������(��ͼ��ʾ)��Сǿ��С��ͬѧ�ܸ���Ȥ����������ɷֽ���̽����
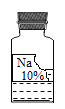
��������⣩��ƿ�Լ�������ʲô��Һ�أ�
���������ۣ����������ǩ������жϣ���ƿ�Լ���������____��(�����)
A �� B �� C ��
���������ϣ�����г������ƵĻ������У�NaCl��NaOH��Na2CO3��NaHCO3��
��Na2CO3��NaHCO3��ˮ��Һ���ʼ��ԡ�
������(![]() )ʱ���ⶨ�������ʵ��ܽ���������£�
)ʱ���ⶨ�������ʵ��ܽ���������£�
���� | NaCl | NaOH | Na2CO3 | NaHCO3 |
�ܽ��g | 36 | 109 | 215 | 9.6 |
С�������Լ�ƿ��ע����������Ϊ10%���ϱ��е��ܽ�������жϣ���ƿ�Լ���������___��
���������룩�ٿ�����NaCl���ڿ�����Na2CO3���ۿ�����____��
��ʵ��̽����(1)Сǿ�ýྻ�IJ�����պȡ��Һ��pH��ֽ�ϣ����pH7������ƿ�Լ���������____��
(2)СǿΪ�˼������Һ��NaOH��Һ����Na2CO3��Һ�����ֽ���������ʵ�顣
ʵ�鲽�� | ʵ������ | ���ۼ���ѧ����ʽ |
��ȡ������Һ���Թ��У��μ�____�� | ������������ | ��Ӧ�Ļ�ѧ����ʽ��___�� |
�ڰѲ���������ͨ������ʯ��ˮ�С� | �����ʯ��ˮ����� | �������ȷ����Ӧ�Ļ�ѧ����ʽ��____�� |
����չ���죩����ѡ����Сǿ��ͬ�����Լ���������NaOH��Һ��Na2CO3��Һ����ѡ����Լ���____��